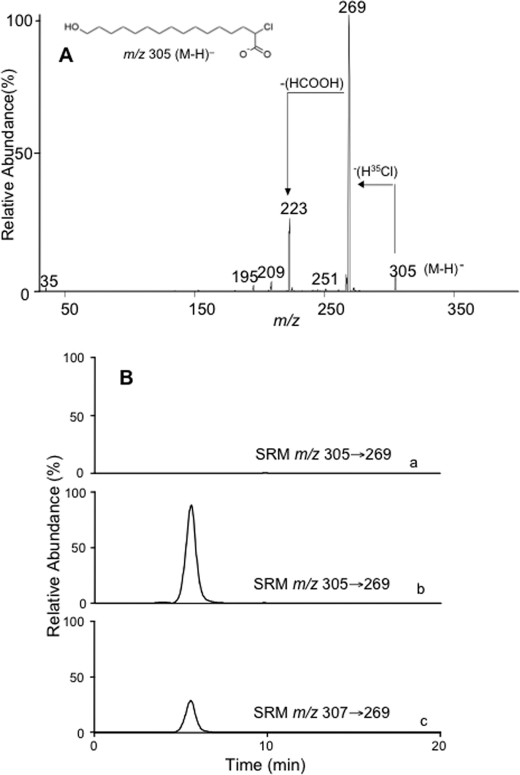
FIGURE 1.
Identification of ω-hydroxy-2-ClHA. 2-ClHA was incubated with liver microsomes for 25 min at 37 °C in the presence or absence of β-NADPH, and reaction products were prepared for analyses as described under “Experimental Procedures.” Product ion spectra of [M − H]− ion of ω-hydroxy-2-35ClHA (m/z 305) obtained by ESI-MS/MS is shown in A. A also shows the prominent neutral losses (marked by arrows) of a putative structure (inset) that is consistent with the starting material (2-ClHA) and the mass spectra. The reaction products were also analyzed by LC-MS/MS (B) as described under “Experimental Procedures.” SRM detection of m/z 305 → 269 for reaction products from incubations in the absence of β-NADPH are shown in trace a. SRM detection of m/z 305 → 269 and m/z 307 → 269 (for ω-hydroxy 2-37ClHA) in the presence of β-NADPH are shown in trace b and c, respectively. In each trace in B, 100% relative abundance was set to equal ion counts.