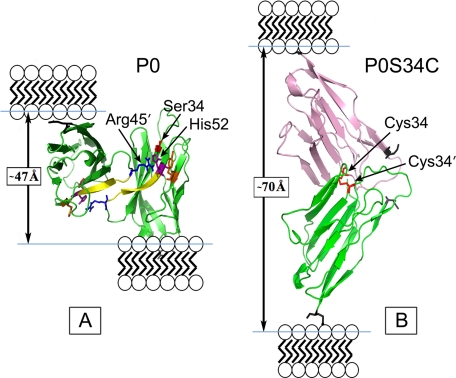
FIGURE 6.
Pymol representation of the WT (A) and modified (B) dimer interface of residues 45–52 of the P0S34C mutant extracellular domain. The WT dimer shows one of the extracellular domains tilted back to reveal better the 2-fold interface. In the WT packing, the distance between the C termini (Glu119, denoted by the black skeletal model) in the membrane stacking direction was measured to be ∼47 Å (arrow). In the swollen myelin, the putative disulfide S34C-S′34C′ dimer gives a distance ∼70 Å between the C termini (arrow) in the membrane stacking direction. Ionizable key residues influencing the electrostatic potential at the dimer interface, including Arg45, His52, and Tyr59, are labeled. The N-terminal Ile1 is indicated by the gray skeletal model. For the modeling, the two P0 molecules were translated and rotated using XtalView so that the Cys34 and Cys34′ were correctly positioned to form a disulfide bond, and then the structure was energy-minimized using the GROMOS96 implementation of Swiss-PdbViewer.