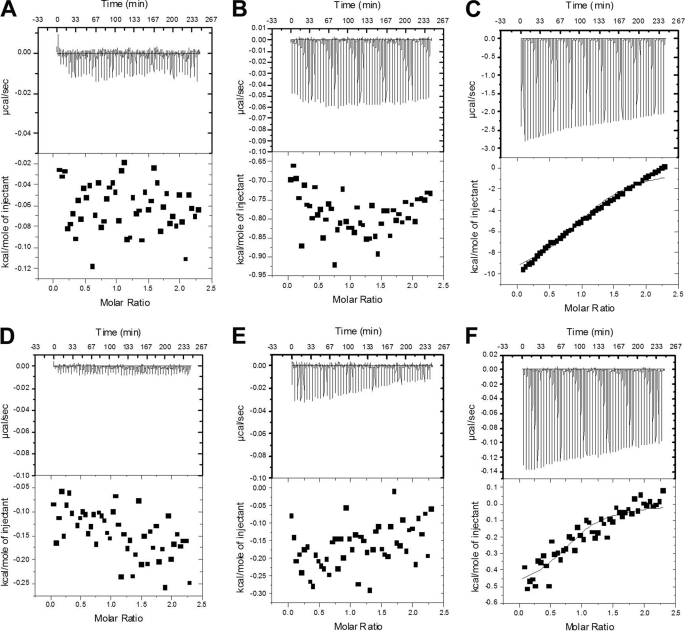
FIGURE 1.
Isothermal titration calorimetry investigation of the influence of glycerol in various buffers in buffer to buffer experiments. ITC was conducted by injecting a specific buffer/glycerol formulation into a sample well containing the same buffer/glycerol formulation. Buffers were: 10 mm phosphate, 100 mm NaCl, 50 mm AMS, pH 6.5 (A); 10 mm phosphate, 100 mm NaCl, 50 mm AMS, 2.5% glycerol (v/v), pH 6.5 (B); 10 mm phosphate, 100 mm NaCl, 50 mm AMS, 5% glycerol (v/v), pH 6.5 (C); ITC Buffer (Falk et al. (3)): 20 mm HEPES/NaOH pH 7.5, 200 mm NaCl, 2 mm MgCl2, 1 mm EDTA, 0.25 mm TCEP (D); ITC Buffer with 2.5% glycerol (Falk et al. (3)): 20 mm HEPES/NaOH pH 7.5, 200 mm NaCl, 2 mm MgCl2, 1 mm EDTA, 0.25 mm TCEP, 2.5% glycerol (v/v) (E); and ITC Buffer with 5% glycerol (Falk et al. (3)): 20 mm HEPES/NaOH pH 7.5, 200 mm NaCl, 2 mm MgCl2, 1 mm EDTA, 0.25 mm TCEP, 5% glycerol (v/v) (F). Although polyols such as glycerol are frequently used to stabilize proteins, they cannot be assumed innocuous.