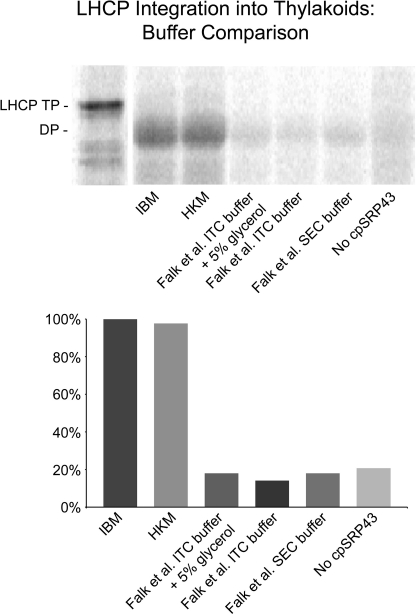
FIGURE 3.
Buffer influence on LHCP integration. Salt-washed thylakoids in IBM were incubated with 5 mm ATP, 1 mm GTP, 12.5 μl of radiolabeled pLHCP translation product, and recombinant cpSRP43, cpSRP54, and cpFtsY (2). The final volume was brought to 150 μl in IBM or to 150 μl with a final concentration matching the buffer listed: 50 mm Hepes/KOH pH 8.0, 330 mm sorbitol, 10 mm MgCl2 (IBM); 10 mm Hepes/KOH pH 8.0, 10 mm MgCl2 (HKM); 20 mm Hepes/NaOH pH 7.5, 200 mm NaCl, 2 mm MgCl2, 1 mm EDTA, 5% (w/v) glycerol, 0.25 mm tris(2-carboxyethyl)phosphine) (ITC Buffer + 5% glycerol (Falk et al. (3)); 20 mm Hepes/NaOH pH 7.5, 200 mm NaCl, 2 mm MgCl2, 1 mm EDTA, 0.25 mm tris(2-carboxyethyl)phosphine) (ITC Buffer (Falk et al. (3)); and 20 mm Hepes/NaOH pH 7.5, 200 mm NaCl, 2 mm MgCl2, 1 mm EDTA, 1 mm DTT (SEC Buffer (Falk et al. (3)). Reactions were incubated at 25 °C for 30 min under light. Membranes were collected by centrifugation at 3200 × g for 6 min at 4 °C and protease-treated with thermolysin. Protease-treated membranes were solubilized in SDS buffer, heated, and analyzed by SDS-PAGE and phosphorimaging. IQ Solutions software (Molecular Dynamics) was used to quantify pLHCP degradation product (indicated as DP), which is indicative of properly inserted LHCP. Each integration assay was compared with the level of integration in IBM (set to 100%). Integration in HKM, used by Lewis et al. (2) for ITC and protein interaction/function assays, is equally as efficient as IBM. Buffers used by Falk et al. (3) do not support integration at a level above the negative control, which lacked cpSRP43.