Figure 1. Voltage- and Ca2+-dependent activation of BK channels.
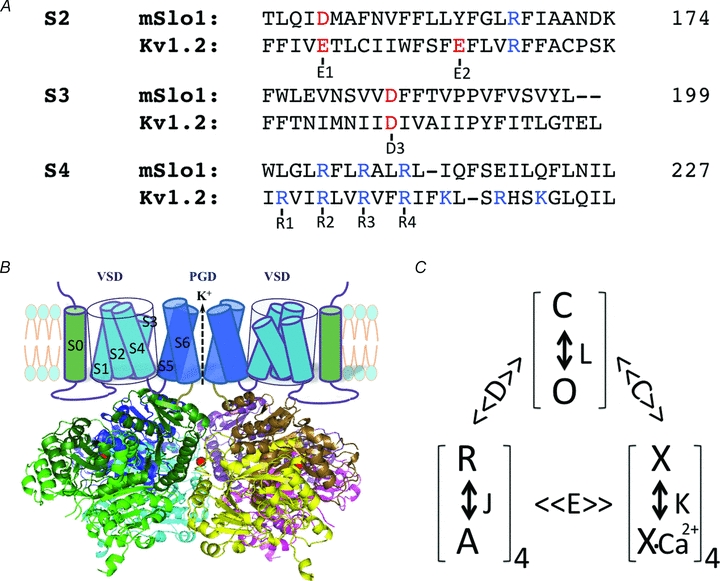
A, sequence alignment of S2–S4 in the voltage sensor domain of mSlo1 and KV1.2 channels, highlighting the conserved charged residues (red and blue) that are important in voltage-dependent activation of both BK and KV channels. The position of the last amino acid in each segment of mSlo1 is given (numbers). B, structure model of BK channels. The cartoon of the membrane-spanning domains only shows two subunits for clarity. The cytosolic domain is the tetrameric Slo1 structure (Yuan et al. 2010). The subunits are represented by different colours and the dark and light shades of each colour show the RCK1 and RCK2 structural domains within the same subunit (RCK: regulator for conductance of K+). A bound Ca2+ ion to the Ca2+ bowl in each subunit is shown as a red sphere. C, model of allosteric gating of BK channels by voltage and Ca2+. The channel can open and close (O and C) with an equilibrium constant L, which is modulated by the activation of four voltage sensors (R and A, with an equilibrium constant J) and the binding of four Ca2+ ions (a simplification from actual binding of eight Ca2+ ions, X and X-Ca2+, with a dissociation constant K) with the allosteric factor D and C, respectively. The voltage sensor activation and Ca2+ binding also affect each other with a factor E. The model was proposed by Horrigan & Aldrich (2002).
