FIGURE 3. A model for the regulation of AMPAR localization.
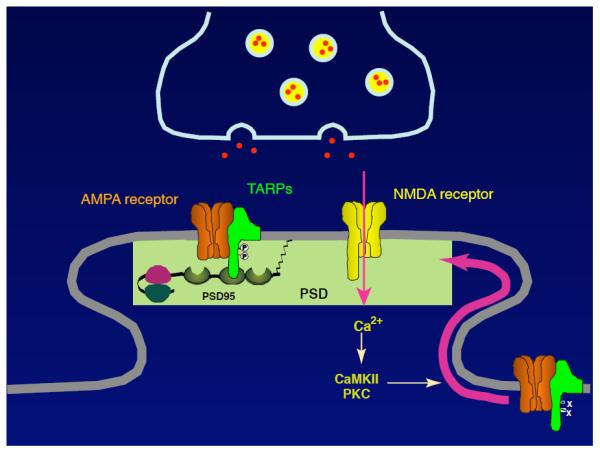
Neuronal activity increases calcium influx through NMDAR to activate calcium-dependent kinases, which is followed by an increase in AMPARs at synapses. TARPs are highly phosphorylated at the PSD. TARP is a substrate of PKC and CaMKII in vitro. These observations indicate that TARP phosphorylation is a regulator of synaptic AMPAR activity and may be a substrate for NMDAR-mediated synaptic plasticity.
