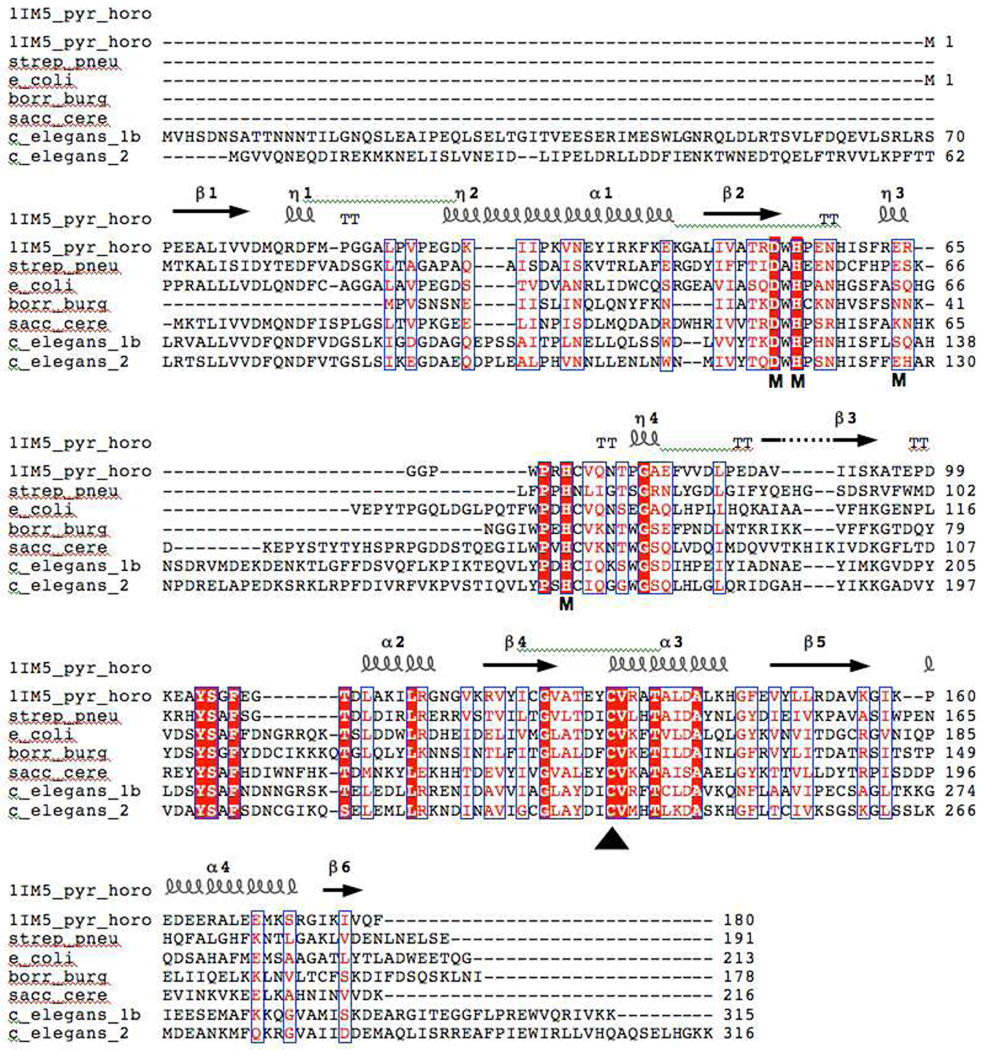
Figure 1.
Multiple sequence alignment of the Pyrococcus horikoshii (PDB entry 1IM5), Streptococcus pneumoniae, Escherichia Coli, Saccharomyces cerevisiae, and Borrelia burgdorferi nicotinamidases. The alignment was performed with ClustalW (56) and edited with ESPript (57). Identical residues are highlighted, and the secondary structure elements of the solved P. horikoshii structure (33) are shown above the alignment. Vertical arrows point to the proposed catalytic triad proposed by Du et al. that is composed of an aspartate, cysteine, and lysine residue. The proposed metal-binding residues are shown with an M under the appropriate residue