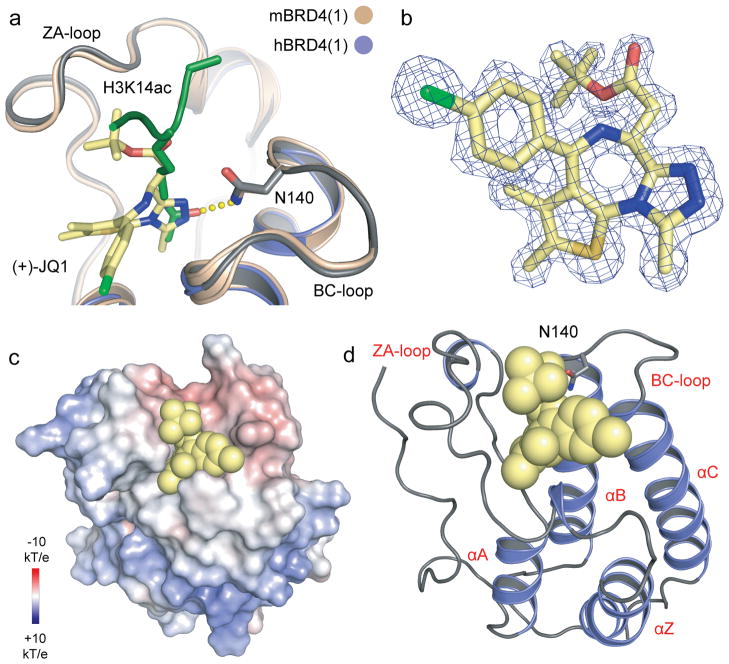
Figure 2. Characterization of BET complexes with (+)-JQ1.
a, Superimposition of the mouse BRD4(1)/H3K14ac peptide complex28 with the human BRD4(1)/(+)-JQ1 complex structure. The hydrogen bond formed to the conserved asparagine (N140) in the peptide complex is shown as yellow dots. b, 2Fo-Fc map of (+)-JQ1 in complex with BRD4(1) contoured at 2σ. c, Electrostatic surface of BRD4(1) in complex with (+)-JQ1. The ligand is shown as a CPK model demonstrating the excellent shape complimentarity with the protein acetylated lysine receptor site. d, Ribbon diagram of the complex of human BRD4(1) with (+)-JQ1 in CPK representation. The main secondary structural elements and the conserved active site asparagine side chain (N140) are labelled.