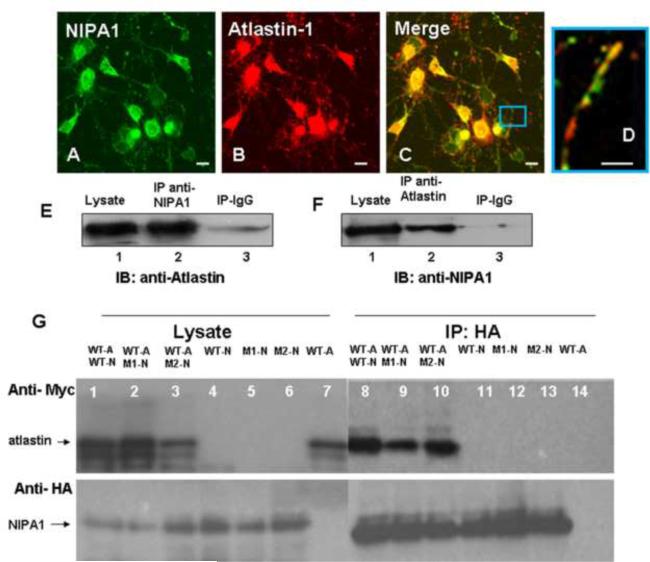
Figure 3. Atlastin-1 and NIPA1 are binding partners.
Staining with anti-NIPA1 antibodies (panel A, green) and anti-atlastin-1 (red, panel B) demonstrated a significant colocalization of endogenous NIPA1 and atlastin-1 proteins in cultured E18 cortical rat neurons (panel C, overlay, panel D – detail of axon). The whole mouse brain extracts proteins were immunoprecipitated (IP) with NIPA1 antibodies or control IgG and analyzed by immunoblotting (IB) with atlastin-1 antibodies (panel E). Similarly, IP with atlastin-1 antibodies and IB with anti-NIPA1 antibodies showed similar results (panel F).
Co-immunoprecipitation of tagged WT atlastin-1-myc (panel G, lanes 1, 2, 3 and 7; upper panel) and NIPA1-HA (lower panel) using anti-HA antibodies from transfected COS-7 cells; lanes 1, 8 and 11 contain WT NIPA1, lanes 2, 9 and 12 T45R NIPA1 mutation, and lanes 3, 6, and 13 G106R NIP1 mutation. Co-immunoprecipitation was observed in lanes 8–10 and NIPA1 mutations did not affect the affinity of both proteins. Similarly, R239C and R495W atlastin-1 mutations did not alter co-immunoprecipitation with WT NIPA1 (data not shown). Lanes 7 and 14 represent a negative control in the absence of NIPA1. (Legend: WT-A = wild type atlastin-1, WT-N = wild type NIPA1, M1-N = T45R NIPA1, M2-N = G106R NIPA1). Scale bar = 5μm.