Figure 5. Possible subunit arrangement of sGC.
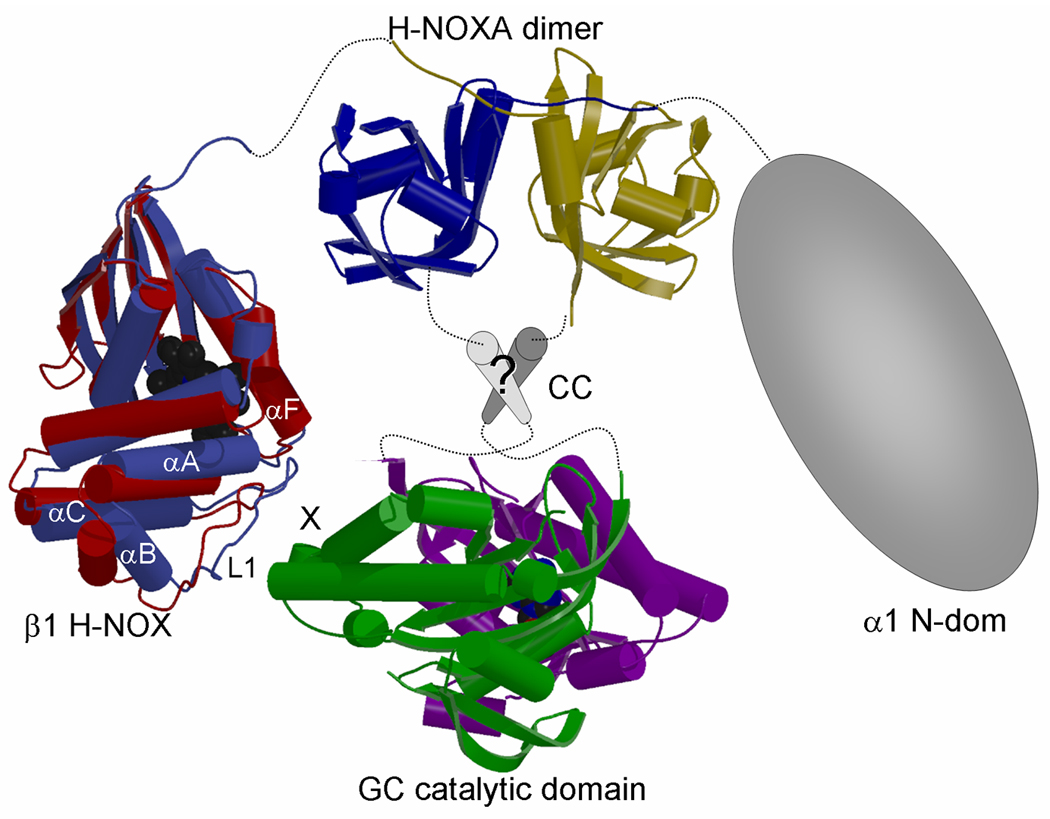
Composite figure depicting possible subunit arrangement of heterodimeric sGC. The structure of the helical putative CC region is not known (labeled ‘?’) as well as the structure of the α1 N-terminal domain. The H-NOX domain is oriented such that helix αF and loop L1 in H-NOX are in proximity to the site that corresponds to where Gsα binds and regulates the homologous adenylyl cyclase (marked ‘X’). In H-NOX, both αF and the N-terminal helical subdomain (αA-αC), which includes the loop L1 containing the potential switch residues D44–D45(14;60), are postulated to shift upon activation (16). To illustrate the N-terminal subdomain shift, we have depicted the superimposed NsH-NOX (red)(16) and TtH-NOX (blue)(14) structures which are postulated to represent the basal and activated state, respectively, of an H-NOX domain. Note that we cannot rule out direct interactions between the H-NOXA domain and the GC catalytic domain, since we do not know the conformation of the intervening sequences and the structure and position of the CC region.
