Table 1.
Synthesis of the macrocyclic tetrazoles[a].
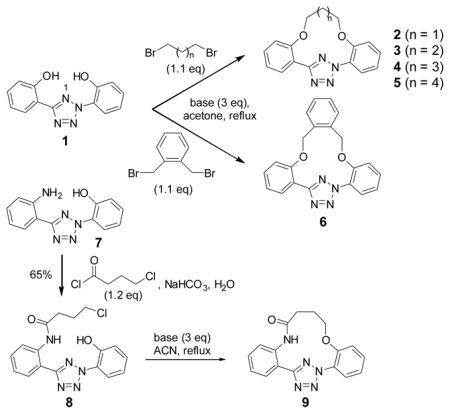 | |||
|---|---|---|---|
| entry | base | product | yield (%)[b] |
| 1 | LiOH•H2O | 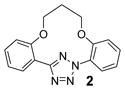 |
trace |
| 2 | Na2CO3 | <5[c] | |
| 3 | K2CO3 | <30[c] | |
| 4 | Cs2CO3 | 38 | |
| 5 | LiOH•H2O | 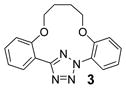 |
trace |
| 6 | Na2CO3 | trace | |
| 7 | K2CO3 | 51 | |
| 8 | Cs2CO3 | 78 | |
| 9 | LiOH•H2O | 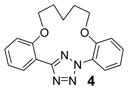 |
trace |
| 10 | Na2CO3 | trace | |
| 11 | K2CO3 | <35[c] | |
| 12 | Cs2CO3 | 45 | |
| 13 | LiOH•H2O | 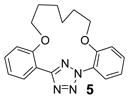 |
trace |
| 14 | Na2CO3 | trace | |
| 15 | K2CO3 | <60[c] | |
| 16 | Cs2CO3 | 67 | |
| 17 | LiOH•H2O | 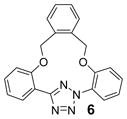 |
trace |
| 18 | Na2CO3 | trace | |
| 19 | K2CO3 | <50[c] | |
| 20 | Cs2CO3 | 61 | |
| 21 | LiOH•H2O | 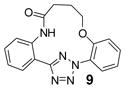 |
trace |
| 22 | Na2CO3 | 70 | |
| 23 | K2CO3 | <20[c] | |
| 24 | Cs2CO3 | trace | |
For bis-O-alkylation, reactions were carried out by refluxing tetrazole 1 with 1.1 equiv of dibromide and 3 equiv of base in acetone overnight. For intramolecular O-alkylation, tetrazole 8 was refluxed in acetonitrile with 3 equiv of base.
Isolated yields were reported unless noted otherwise.
Estimated yields based on TLC.
