Figure 7.
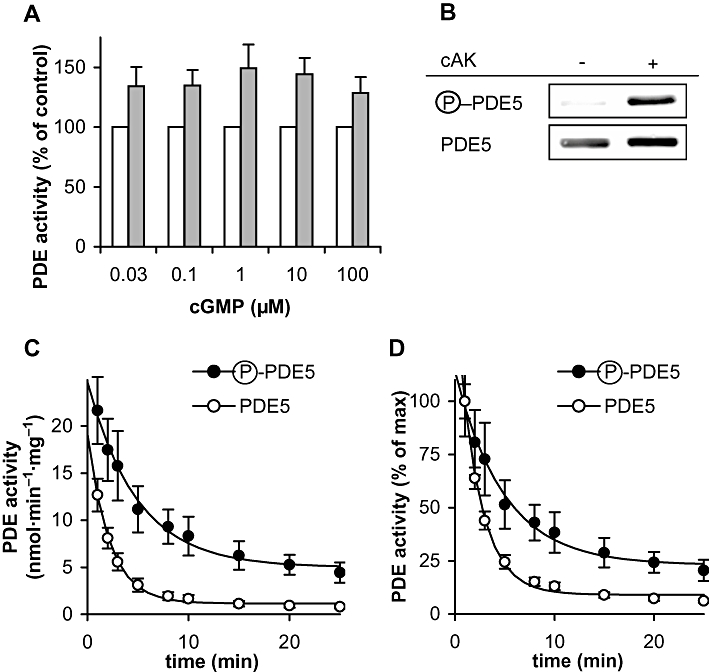
Phosphorylated PDE5 shows higher catalytic rates and a slower deactivation. PDE5 was phosphorylated as described in detail in the Methods. (A) Comparison of activity of phosphorylated versus non-phosphorylated PDE5 at the indicated concentrations of cGMP as substrate. The reactions were carried out as described in detail in Methods. P < 0.05 in paired t-test for phospho- versus non-phospho-PDE5 at all substrate concentrations. (B) Aliquots were analysed in Western blots with antibodies specifically detecting phosphorylated PDE5 and antibodies against PDE5 to check loading. The blot shown is representative of four performed with similar results (full lanes of the blots are depicted in Figure S4). cAK, cAMP-dependent protein kinase (C) PDE5 deactivation was monitored by measuring PDE activity of phosphorylated versus non-phosphorylated PDE5 in 10 s incubations at the indicated time points after a 1000-fold dilution to reduce the GAF ligand 8-Br-cGMP to concentrations below 0.3 µM. (D) Data from (C) normalized to the 1 min value. Data shown are mean ± SD of at least three independent experiments performed in duplicate.
