Abstract
BACKGROUND AND PURPOSE
Intracerebroventricularly injected tachykinin NK3 receptor (R) antagonists normalize mean arterial blood pressure (MAP) in spontaneously hypertensive rats (SHR). This study was pursued to define the role played by NK3R located on dopamine neurones of the ventral tegmental area (VTA) in the regulation of MAP in SHR.
EXPERIMENTAL APPROACH
SHR (16 weeks) were implanted permanently with i.c.v. and/or VTA guide cannulae. Experiments were conducted 24 h after catheterization of the abdominal aorta to measure MAP and heart rate (HR) in freely behaving rats. Cardiovascular responses to i.c.v. or VTA-injected NK3R agonist (senktide) and antagonists (SB222200 and R-820) were measured before and after systemic administration of selective antagonists for D1R (SCH23390), D2R (raclopride) or non-selective D2R (haloperidol), and after destruction of the VTA with ibotenic acid.
KEY RESULTS
I.c.v. or VTA-injected SB222200 and R-820 (500 pmol) evoked anti-hypertension, which was blocked by raclopride. Senktide (10, 25, 65 and 100 pmol) elicited greater increases of MAP and HR when injected in the VTA, and the cardiovascular response was blocked by R-820, SCH23390 and haloperidol. VTA-injected SB222200 prevented the pressor response to i.c.v. senktide, and vice versa, i.c.v. senktide prevented the anti-hypertension to VTA SB222200. Destruction of the VTA prevented the pressor response to i.c.v. senktide and the anti-hypertension to i.c.v. R-820.
CONCLUSIONS AND IMPLICATIONS
The NK3R in the VTA is implicated in the maintenance of hypertension by increasing midbrain dopaminergic transmission in SHR. Hence, this receptor may represent a therapeutic target in the treatment of hypertension.
Keywords: dopamine, tachykinins, NK3R, senktide, SB222200, R-820, ventral tegmental area, hypertension, spontaneously hypertensive rats
Introduction
The ventral tegmental area (VTA), the origin of the mesolimbic and mesocortical (A10) dopaminergic pathways, is traditionally known for its implication in the regulation of locomotor activity, stress responses, reinforcement and reward mechanisms (Le Moal and Simon, 1991; Swanson et al., 2000; Hyman and Malenka, 2001). The literature from the 1990s suggests a role for the VTA and the mesolimbic dopaminergic system in cardiovascular homeostasis in link with the defence reaction and the somatosensory input (Van den Buuse, 1998). The mesolimbic dopaminergic system is involved in diurnal blood pressure (BP) and heart rate (HR) regulation (Sei et al., 1999; Sakata et al., 2002). The discharge rate of dopaminergic neurones in the VTA seems to be regulated by inputs from arterial baroreceptors, probably through a relay in the nucleus tractus solitarius (Kirouac and Ciriello, 1997). The stimulation of the VTA is also associated with the modulation of the circulatory and pressor effects of vasopressin (Van den Buuse and Catanzariti, 2000). Electrical stimulation of the rat VTA or microinjection of a substance P (SP) analogue produces increases of BP and HR through the activation of dopamine D1 and D2 receptors (R), and vasopressin release (Cornish and Van den Buuse, 1995).
In a further recent study, the VTA was proposed as a potential target for tachykinin receptors in cardiovascular regulation (Deschamps and Couture, 2005). Increases of BP, HR and stereotypic behaviours are elicited by the intra-VTA injection of physiological doses (pmol) of tachykinin NK1, NK2 and NK3 receptor agonists in freely moving rats. These responses are blocked in a selective manner by their respective antagonist and by systemic treatment with the D1R antagonist, SCH23390, but not with the D2R antagonist, raclopride. Furthermore, the cardiovascular response to tachykinin agonists is ascribed to enhanced cardiac output following the stimulation of cardiac β1-adrenoceptors. This study is consistent with the increased firing rate of A10 dopamine (DA) cells induced by the application of NK1, NK2 and NK3 receptor agonists in the VTA (Overton et al., 1992), and the presence of NK1 and NK3 receptors on dopaminergic and non-dopaminergic neurones in the VTA as revealed by confocal and electron microscopy (Chen et al., 1998; Lessard et al., 2007; 2009;). The VTA is rich in NK3 binding sites (Dam et al., 1990; Stoessl and Hill, 1990; Ding et al., 1996; Langlois et al., 2001) and is particularly sensitive to the NK3R agonist senktide (Seabrook et al., 1995; Deschamps and Couture, 2005). SP and senktide injected into the VTA enhance levels of DA and its metabolite dihydroxyphenylacetic acid, DA turnover in the prefrontal cortex and nucleus accumbens (Elliott et al., 1986; Cador et al., 1989; Overton et al., 1992; Marco et al., 1998), and behaviour consistent with mesolimbic DA activation (Eison et al., 1982; Elliott and Iversen, 1986; Deschamps and Couture, 2005). SP, neurokinins A and B are found in the VTA (Deutch et al., 1985; Kalivas et al., 1985; Warden and Young, 1988), and SP immunoreactive axon terminals make direct synaptic contact with DA neurones in the VTA (Tamiya et al., 1990). SP projecting fibres in the VTA originate from the nucleus accumbens and the habenular nucleus (Cuello et al., 1978; Lu et al., 1998).
In a recent study, we reported that i.c.v. injection of NK3R antagonists reverses hypertension in spontaneously hypertensive rats (SHR) and does not affect resting blood pressure in Wistar-Kyoto rats (WKY) (Lessard et al., 2004). Conversely, the inhibition of brain NK1R and NK2R fails to affect BP in SHR and WKY. Whereas these findings support a role for brain tachykinin NK3R in the maintenance of hypertension in SHR, the underlying mechanism of the anti-hypertensive effect of NK3R antagonists remains elusive. We showed that it persists after bilateral nephrectomy and is not accompanied by changes in plasma levels of vasopressin (Lessard et al., 2004).
Based on all these revised findings, the VTA may represent a strategic site for the central anti-hypertensive effect of NK3R antagonists in SHR. This is in keeping with the increased central DA activity, which contributes substantially to the hypertension in SHR (Van den Buuse, 1997; Amenta et al., 2001). An up-regulation of D1R and D2R in the nucleus accumbens, one major neuronal projection of the VTA, was reported in young SHR (Kirouac and Ganguly, 1993; Vaughan et al., 1999; Russell, 2003).
The present pharmacological study was undertaken to test the hypothesis that tachykinin NK3R in the VTA contributes to the maintenance of high arterial BP by interacting with the mesocorticolimbic dopaminergic system in freely behaving SHR. This was achieved by determining the cardiovascular response to the selective NK3R agonist (senktide) and antagonists (SB222200 and R-820) injected i.c.v., or into the VTA, before and after inhibition of D1R and D2R (with SCH23390 and raclopride, respectively) or after destruction of the VTA with ibotenic acid (IBO). Data suggest that tachykinin NK3R located in the VTA is subjected to persistent tonic activation, which increases midbrain dopaminergic transmission and thereby contributes to hypertension in SHR.
Methods
Animal source and care
Male SHR (16 weeks) were purchased 1 week prior to experiments from Charles River (St. Constant, Quebec, Canada). They were housed two per cage under a 12 h light–dark cycle in a room with controlled temperature (22°C) and humidity (40%). Food (Charles River Rodent) and tap water were available ad libitum. The care of animals and research protocols conformed to the guiding principles for animal experimentation as enunciated by the Canadian Council on Animal Care and approved by the Animal Care Committee of our University.
Animal preparation
Before each surgery, rats received the antibiotics trimethoprim and sulphadiazine (Tribrissen 24%, 30 mg kg−1, s.c., Schering Canada Inc., Pointe Claire, Quebec, Canada) and the analgesic ketoprophen (anafen, 10 mg kg−1, s.c., Merial Canada Inc., Baie d'Urfé, Quebec, Canada). They were anaesthetized with isoflurane (3%) and then positioned in a stereotaxic apparatus (David Kopf Instrumentation, Tujunga, CA, USA) with the incisor bar set at 0.0 mm in the interaural line for i.c.v and at 3.3 mm below the interaural line for VTA implantation. The skull was exposed, cleaned and a hole was drilled to implant a 23-gauge stainless-steel guide cannula into the left (contralateral) or right (ipsilateral) lateral brain ventricle (coordinates: 0.6 mm posterior to the bregma, 1.3 mm lateral to the midline, 3.0 mm ventral from the skull surface), and/or 2 mm above the left or right VTA (coordinates: 5.3–5.6 mm posterior to the bregma, 0.7 mm lateral to the midline, 7.0 mm ventral from the skull surface) according to Paxinos and Watson (1998). The guide cannula was fixed with two screws and dental cement to the skull. Then the skin was replaced and sutured. Finally, a stylet (31-gauge stainless-steel) was inserted into the guide cannula to prevent loss of CSF and to avoid its obstruction. Animals were housed in individual plastic cages (40 × 23 × 20 cm) in the same controlled conditions and allowed to recover.
Three days later, the animals were re-anaesthetized with isoflurane and two siliconized PE-10 catheters (one PE-10 connected to PE-60), filled with physiological saline and 100 iu mL−1 heparin sodium salt, were inserted into the abdominal aorta through the left femoral artery for BP recording and into the left femoral vein for drug administration respectively. They were passed subcutaneously to emerge at the back of the neck and secured with tape. During the following days, the animals were observed closely. Those which lost more than 20% of their body weight or had clear signs of cerebral haemorrhage, atypical behaviour or weaknesses were killed with CO2 inhalation. Experimental protocols were initiated 24–48 h after the last vascular surgery in conscious and freely moving rats (Deschamps and Couture, 2005). Successful VTA and i.c.v. implantation were confirmed in all rats used in the study after post-mortem examination.
Measurement of cardiovascular parameters
During all experiments, continuous direct recordings of arterial BP and HR were made on a high-performance data acquisition system PowerLab 8/30 with LabChart Pro ML870P and ML228 Bridge Amp (ADInstruments Inc, Colorado Springs, CO, USA). Mean arterial blood pressure (MAP) was calculated from systolic (S) and diastolic (D) blood pressure values [(S − D) ÷ 3 + D = MAP].
The cardiovascular response was measured 1 h after the rats were transported to an isolated and quiet testing room. Rats remained in their resident cage, but the top grid containing the food and tap water was removed prior to and up to 1 h post-injection. When BP and HR were stable, a 31-gauge stainless-steel injector connected to a 5 µL Hamilton microsyringe via a PE-10 tubing catheter was inserted into the guide cannula, which was accessible without handling the rat. Five minutes later, injection was made i.c.v. or directly into the VTA of undisturbed, freely moving rats. All solutions were freshly prepared and injected (volume of 1 µL i.c.v.; 0.5 µL VTA and 0.1 mL kg−1 i.v.) over a period of 1 min. The injector was removed from the guide cannula 1 min after injection to prevent any possible leakage of the injectate.
Experimental protocols
Cardiovascular effects of i.c.v and VTA administered tachykinin NK3R antagonists
This series of experiments was aimed at determining the anti-hypertensive effect of centrally administered NK3R antagonists (500 pmol). SHR were administered the selective tachykinin NK3R antagonist i.c.v. (SB222200, n = 8) (Sarau et al., 2000) 1 h after the vehicle [artificial cerebrospinal fluid (aCSF), n = 8]. The inactive enantiomer of SB222200 (SB222201, n = 10) was also injected as control for specificity in a separate group of SHR. The selection of this dose of SB222200 was based on a previous dose-response curve, which established that 500 pmol SB222200 i.c.v. caused the optimal anti-hypertensive effect in SHR (Lessard et al., 2004). For comparison, SHR implanted with a VTA cannula were administered aCSF and 1 h later SB222200 (500 pmol, n = 5).
In two separate groups of SHR, R-820 (500 pmol) was injected either i.c.v. (n = 5) or directly into the VTA (n = 4), and the anti-hypertensive responses were measured for up to 3 days or until they were back to baseline values. The dose and duration of recording were based on a previous i.c.v. study done in SHR (Lessard et al., 2004).
Effects of systemic treatments with the D1 and D2 receptor antagonists against SB222200 and R-820
This series of experiments was designed to determine the contribution of DA and its receptors to the anti-hypertensive effects of NK3R antagonists. SHR that have received SB222200 (500 pmol, 24 h previously) or R-820 (500 pmol, 72 h previously) by the i.c.v. or the VTA route were given the D1R antagonist SCH23390 (0.2 mg kg−1, i.v.) (Kirouac and Ciriello, 1997; Gioanni et al., 1998) or the D2R antagonist raclopride (0.16 mg kg−1, i.v.) (Millan et al., 1998) 30 min prior to a second injection of the NK3R antagonist. SB222200 and R-820 (500 pmol) were re-injected again either 24 h (SB222200) or 72 h (R-820) later to assess whether any blockade observed in the presence of the DA antagonist could be reversed. Each rat was injected with only one DA antagonist and one NK3R antagonist either i.c.v or into the VTA.
Chronic anti-hypertensive effect of i.c.v. R-820
SHR (n = 4) were injected daily with R-820 (500 pmol, i.c.v.) to see whether its anti-hypertensive effect could be maintained for a period of 5 days. MAP and HR were measured at the peak time within the 5 h post-injection period every day. The recording was pursued for up to 2 days after cesssation of the treatment.
Dose–response curves to i.c.v. or VTA microinjection of the NK3R agonist senktide
Two groups of SHR were used on 4 consecutive days; the first group was implanted with an i.c.v. cannula (n = 6) and the second group with a VTA cannula (n = 6). Each group was administered aCSF, either i.c.v. or directly into the VTA, followed by 10 pmol senktide 60 min later, and then increasing doses of 25, 65 and 100 pmol were administered on the following 3 days at 24 h intervals. This protocol and the doses selected were based on our previous studies in which the cardiovascular effects of senktide, injected either i.c.v. or into the VTA, were characterized in normotensive rats (Cellier et al., 1997; Deschamps and Couture, 2005).
Blockade of the effects of senktide by the tachykinin NK3R antagonist in the VTA
In this series of experiments, the dose of 25 pmol senktide was selected on the basis of its maximal cardiovascular response after VTA injection. SHR (n = 4) were administered a microinjection of aCSF into the VTA and followed 60 min later by a single dose of senktide (25 pmol). Twenty-four hours later, senktide was re-injected again 1 h after the VTA injection of the tachykinin NK3R antagonist R-820 (500 pmol) (Regoli et al., 1994). The reversal of the inhibition was tested 24 h later.
Effects of systemic treatments with dopamine D1 and D2 receptor antagonists against i.c.v. senktide
In this series of experiments, the dose of 65 pmol senktide was selected on the basis of its maximal pressor response upon i.c.v. injection. Three groups of SHR were initially injected i.c.v. with a single dose of senktide (65 pmol). One hour later, they were injected with either the D1R antagonist SCH23390 (0.2 mg kg−1, i.v. n = 6), the D2R antagonist raclopride (0.16 mg kg−1, i.v. n = 6) or the non-selective D2R antagonist haloperidol (10 mg kg−1, s.c. n = 4) (Nsimba et al., 1997; Miyamoto et al., 2005), followed 30 min later by senktide (65 pmol). The following day (24 h later), senktide (65 pmol) was re-injected alone to assess the reversal of any blockade observed in the presence of antagonist on the preceding day. In one additional group of SHR (n = 4), raclopride and SCH23390 were administered alone, 24 h apart, to assess their direct cardiovascular effects. A second group of SHR (n = 4) served to evaluate the direct cardiovascular effect of haloperidol. Pilot experiments have shown that the cardiovascular response to i.c.v. senktide (65 pmol) is reproducible when two injections are given at 1 h apart.
Blockade of i.c.v. senktide by SB222200 injected into the VTA of SHR and reciprocally blockade of VTA SB222200 by i.c.v. senktide
This experiment aimed to determine, firstly, the contribution of VTA NK3R to the i.c.v. pressor response to senktide, and secondly, the possibility that the anti-hypertensive effect of SB222200 injected into the VTA could be prevented by an ipsilateral i.c.v. injection of the agonist senktide. Rats were injected with senktide (65 pmol, i.c.v. n = 4) (right side). Two hours later, SB222200 (500 pmol) was injected into the ipsilateral VTA, and senktide was re-injected i.c.v. 1 h later to evaluate the inhibition of the pressor response to senktide. On the second day, SB222200 (500 pmol, n = 4) was injected into the right VTA to assess its anti-hypertensive effect. On the third day, senktide (65 pmol) was injected i.c.v. (right side) 1 h after injection of SB222200 into the right VTA to see if it could block the anti-hypertensive effect of SB222200, which starts 1 h post-injection. In another group of rats (n = 4), senktide was injected i.c.v. on the contralateral side (left side) of VTA injection of SB222200 (right side) and the rest of the protocol was similar to that described previously.
Effect of ipsilateral and contralateral lesions of the VTA with IBO on the cardiovascular response to senktide and R-820
IBO has been shown to be an effective agent for producing circumscribed lesions when local, discrete brain lesions are desired. This method works reliably without apparent damage to passing or underlying fibres and, in contrast to kainic acid, a similar excitatory amino acid toxin, without causing distant lesion effects in the brain (Guldin and Markowitsch, 1981; 1982;).
Senktide (65 pmol) was given i.c.v. to two groups of sham-operated rats (n = 4) and to rats subjected to ipsilateral (n = 4) or contralateral (n = 4) lesions of the VTA, induced by administration of IBO (1 µg in 0.5 µL) injected directly into the VTA, 5 days earlier. Twenty-four hours after senktide injection, R-820 (500 pmol) was given i.c.v. to the four groups of SHR.
Histology
At the end of the experimental protocol, the rats were killed by CO2 inhalation and then immediately injected with 0.5 µL of Evans Blue dye (Sigma-Aldrich Canada Ltd, Oakville, Ontario, Canada) in the VTA. The brains were removed and fixed with 10% (vv−1) buffered formalin (Fisher Scientific Inc., Ontario, Canada) and 20% (wv−1) sucrose until the piece was floating (around 2 weeks) on the surface (Carson, 1992). Coronal sections (40 µm, cut on a freezing microtome) were mounted on glass slides and stained with cresyl violet for histological examination of the microinjection site. The injection site was confirmed by the presence of a black spot in the VTA with no evidence of haemorrhage or necrosis (Figure 1).
Figure 1.
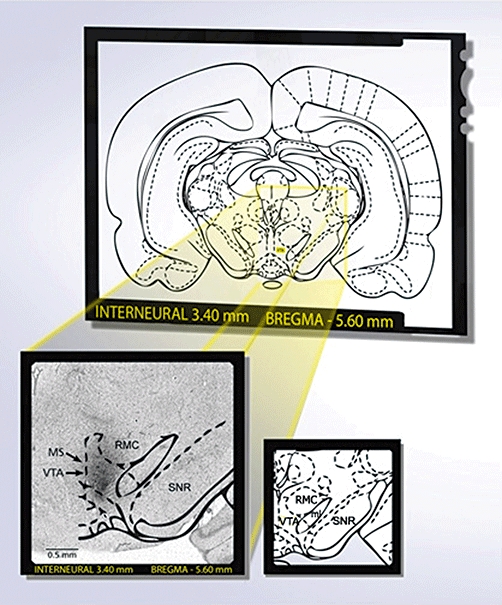
Identification of the VTA as a microinjection site following post-mortem histological examination of microinjected Evans's blue. A rat was considered to be correctly injected when a black spot was seen in the VTA without any evidence of haemorrhage or necrosis. Diagram was modified from the atlas of Paxinos and Watson (1998). VTA, ventral tegmental area; SNR, substantia nigra reticular; ml, medial lemniscus; RMC, red nucleus magnocellular; MS, microinjection site. Scale bar: 0.5 mm.
Drugs and solutions
The drug target nomenclature conforms to the British Journal of Pharmacology's Guide to Receptors and Channels (Alexander et al., 2009). aCSF was purchased from Harvard Bioscience (Holliston, MA, USA.). Succinyl-[Asp6,MePhe8]-SP (6–11) (senktide) (MW: 842) was purchased from Bachem Bioscience Inc. (King of Prussia, PA, USA). Raclopride, haloperidol, IBO and heparin were purchased from Sigma-Aldrich Canada and SCH23390 from Tocris (Ellisville, MO, USA). Haloperidol was dissolved in ethanol and dimethylsulphoxide (DMSO), and diluted in saline (final solution contained 3% ethanol and 0.5% DMSO). SCH23390 and raclopride were dissolved in DMSO and diluted in saline (final solutions contained 3% DMSO). R-820 (3-indolylcarbonyl-Hyp-Phg-N (Me)-Bzl) (MW: 624.6) was a kind gift of the late Dr Jean-Luc Fauchère (Research Institute Servier, Paris, France) (Regoli et al., 1994). SB222200 [(S)-(–)-N-(α-ethylbenzyl)-3-methyl-2-phenylquinoline-4-carboxamide (MW: 686.7) and its inactive enantiomer SB222201 were a donation from Dr Henry M. Sarau (GlaxoSmithKline, PA, USA.) (Sarau et al., 2000). They were dissolved in DMSO and aCSF mixed with 2-hydroxypropyl-β-cyclodextrin (Sigma-Aldrich Canada) to obtain the desired solution (final solutions contained less than 15% DMSO and 20% 2-hydroxypropyl-β-cyclodextrin).
Statistical analysis of data
Results are expressed as the means ± SEM of values obtained from (n) rats. Statistical analysis of data was performed with Graph-Pad Prism software (GraphPad Software Inc., La Jolla, CA, USA). Time-course effects were analysed for statistical significance by a two-way analysis of variance (anova) followed by a Bonferroni's test for multiple comparisons. A one-way anova followed by a post hoc Dunnett's test was used for multiple comparisons with the same control group or by a post hoc Bonferroni's test for comparison between groups [area under the curve (AUC)]. Only probability values (P) less than 0.05 were considered to be statistically significant.
Results
Cardiovascular responses induced by i.c.v. or VTA-injected SB222200
The tachykinin NK3R antagonist SB222200 (500 pmol) injected i.c.v. caused a significant anti-hypertensive effect from 5 to 10 h post-injection in comparison with vehicle values in SHR. MAP was back to hypertensive levels (184.1 ± 3.8 mm Hg) 24 h later (Figure 2A). The anti-hypertension induced by SB222200 peaked at 8 h (−42 ± 5.5 mm Hg, P < 0.001) and was associated with a significant bradycardia. In contrast, the inactive enantiomer of SB222200 (SB222201) did not affect MAP or HR in comparison with vehicle values.
Figure 2.
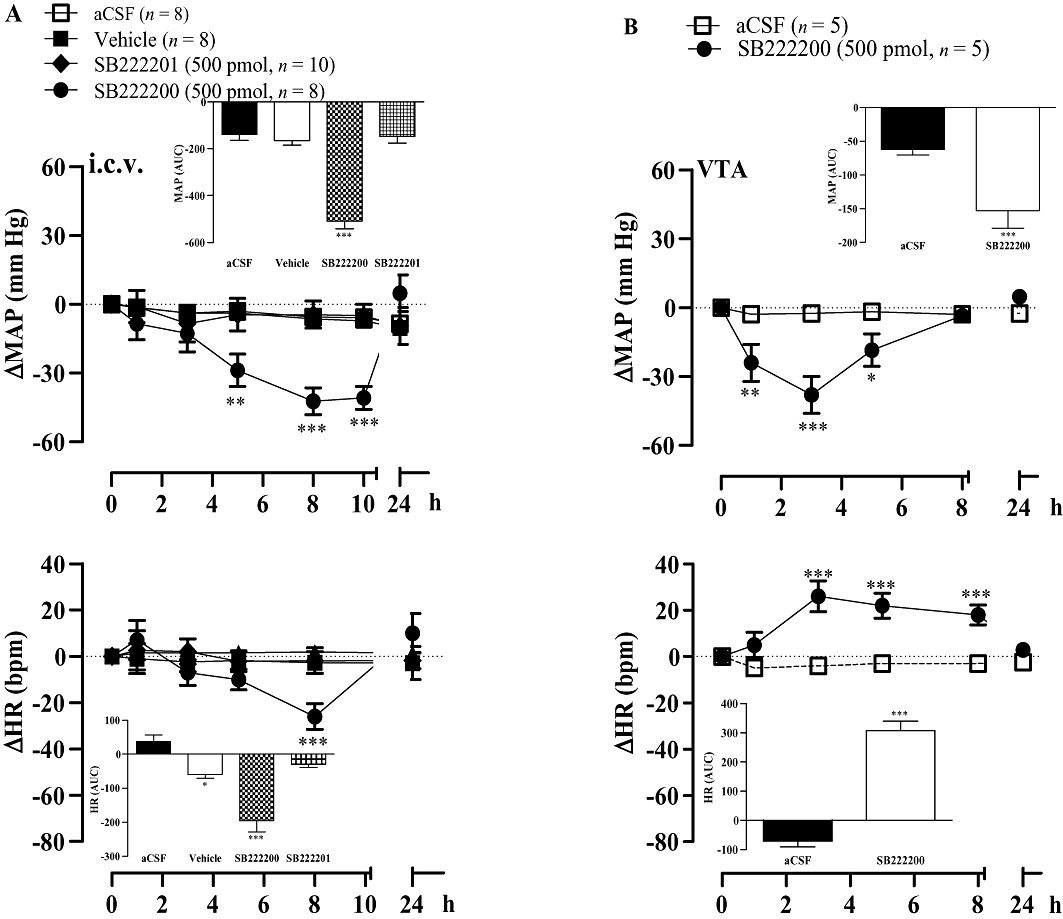
Time-course effects on changes in mean arterial blood pressure (ΔMAP) and heart rate (ΔHR) induced by i.c.v. (A) or ventral tegmental area (VTA) (B) injection of 500 pmol of SB222200 or its inactive enantiomer SB222201. Areas under the curves (AUC) were measured for a period of 0–10 h for i.c.v and 0–8 h for VTA (small insets). Values represent the mean ± SEM of n rats. Significant difference from aCSF is indicated by *P < 0.05; **P < 0.01; ***P < 0.001.
The injection of SB222200 (500 pmol) into the VTA caused a more rapid decrease in MAP, which was significant at 1 h and peaked at 3 h post-injection in SHR. This anti-hypertensive effect of the NK3R antagonist was resolved at 8 h and was associated with a significant increase in HR at 3 h and during the remaining recording period of 8 h (Figure 2B).
Effects of dopamine antagonists on anti-hypertensive responses induced by tachykinin NK3R antagonists
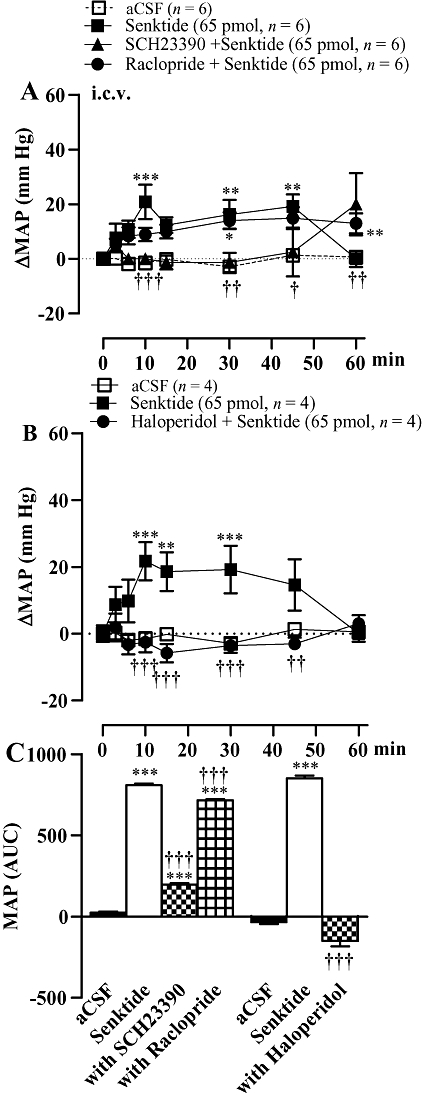
Raclopride (0.16 mg kg−1, i.v.) prevented the anti-hypertensive effect of i.c.v. SB222200 (500 pmol) and reduced the accompanying bradycardia (Figure 3A). Conversely, SCH23390 (0.2 mg kg−1, i.v.) caused a small reduction in the cardiovascular response to SB222200 (Figure 3A). The anti-hypertensive effect mediated by i.c.v. SB222200 was completely recovered 24 h later when it was re-injected in the absence of DA antagonists (data not shown). Likewise, the longer-lasting anti-hypertensive response and bradycardia induced by i.c.v. R-820 (500 pmol) were blocked by raclopride (0.16 mg·kg−1, i.v.) (Figure 3B). A similar treatment with raclopride also prevented the more rapid, although shorter-lasting, anti-hypertensive response and the accompanying tachycardia elicited by R-820 injected into the VTA (Figure 4).
Figure 3.
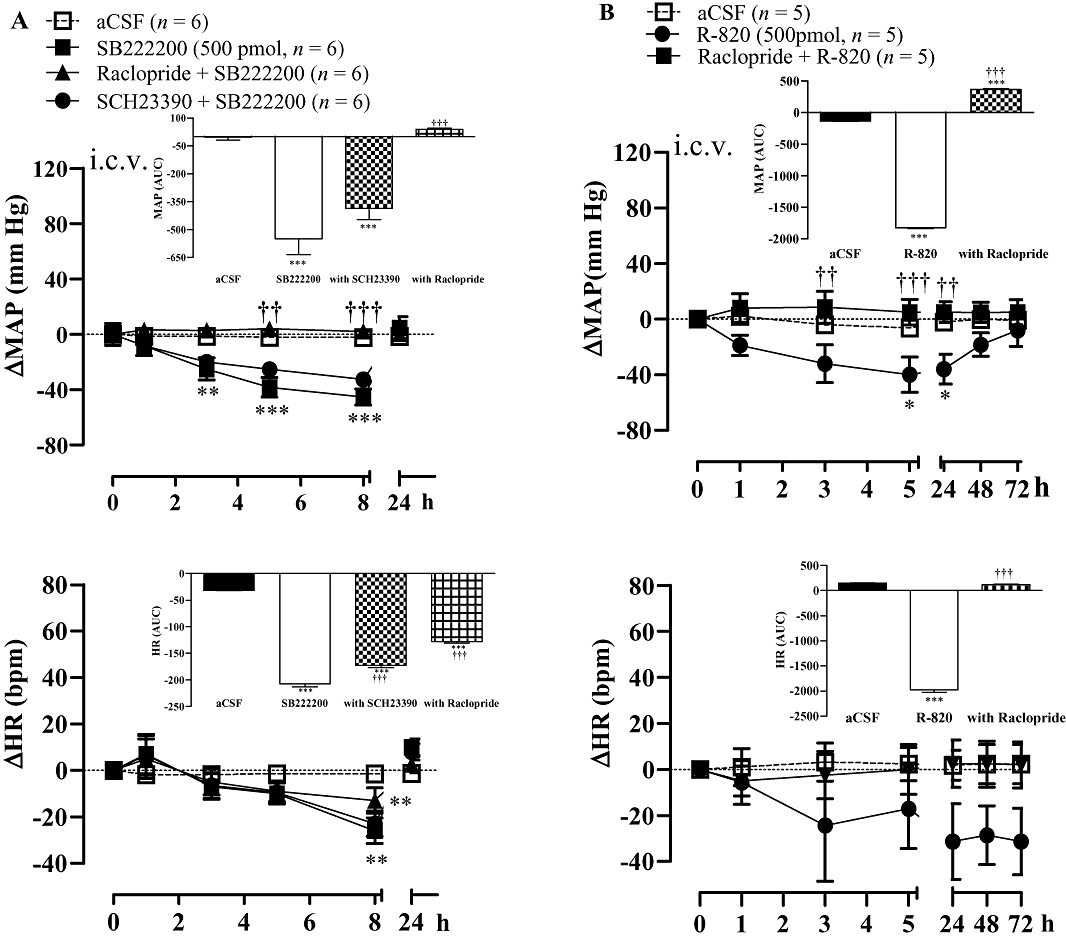
Time-course effects on changes in mean arterial blood pressure (ΔMAP) and heart rate (ΔHR) induced by i.c.v. injection of (A) SB222200 (500 pmol) and (B) R-820 (500 pmol) before and 30 min after treatment with the D2R antagonist raclopride (0.16 mg kg−1, i.v.) or the D1R antagonist SCH23390 (0.2 mg kg−1, i.v.). Areas under the curves (AUC) were measured for a period of 0–8 h (SB222200) or 0–72 h (R-820) as shown in small insets. Values represent the mean ± SEM of n rats. Significant difference from aCSF values (*) or NK3R antagonist alone (†) is indicated by *P < 0.05, **††P < 0.01; ***†††P < 0.001.
Figure 4.
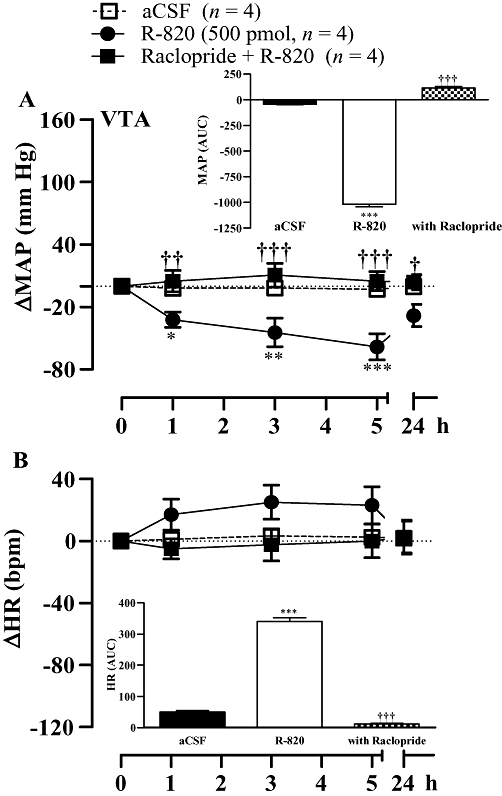
Time-course effects on changes in mean arterial blood pressure (ΔMAP) (A) and heart rate (ΔHR) (B) induced by R-820 (500 pmol) injected into the ventral tegmental area (VTA) before and 30 min after treatment with the D2R antagonist raclopride (0.16 mg kg−1, i.v.). Areas under the curves (AUC) were measured for a period of 0–5 h (small insets). Values represent the mean ± SEM of n rats. Significant difference from aCSF values (*) or R-820 (†) is indicated by *†P < 0.05; **††P < 0.01; ***†††P < 0.001.
Chronic anti-hypertensive effect of i.c.v. R-820
In SHR, daily i.c.v. injection of R-820 (500 pmol) for 5 days led to a persistent decrease of both MAP and HR in comparison with the pre-administration values. MAP and HR values returned gradually to baseline hypertensive values within 48 h after cessation of the treatment with R-820 (Figure 5).
Figure 5.
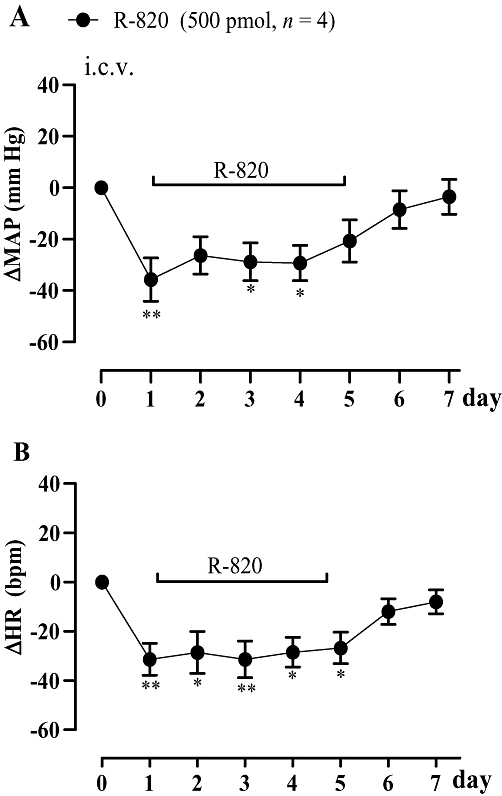
Time-course effects on changes in mean arterial blood pressure (ΔMAP) and heart rate (ΔHR) induced by i.c.v. injection of 500 pmol R-820, once daily for a period of 5 days in spontaneously hypertensive rats. Values represent the mean ± SEM of four rats. Significant difference from pre-injection values is indicated by *P < 0.05; **P < 0.01.
Comparison of the cardiovascular effect of i.c.v and VTA injected senktide
The i.c.v. injections of the tachykinin NK3R agonist senktide (10–100 pmol) increased MAP and HR significantly but not dose-dependently during the first 30–60 min post-injection in SHR. The pressor response was maximal at 65 pmol, while the tachycardiac response was greater in intensity and duration at 25 pmol (Figure 6A).
Figure 6.
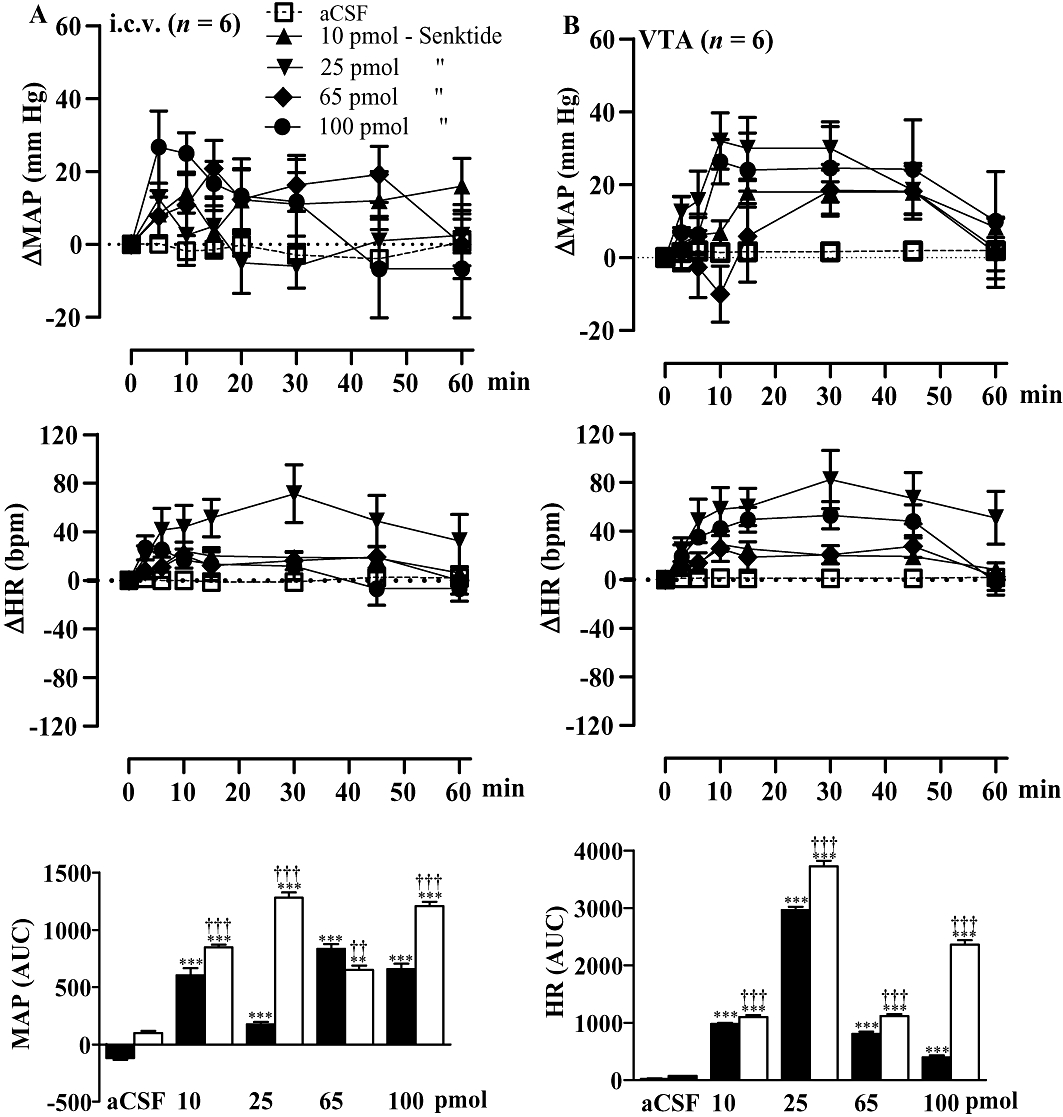
Time-course effects on changes in mean arterial blood pressure (ΔMAP) and heart rate (ΔHR) following i.c.v. (A) or ventral tegmental area (VTA) (B) injection of four increasing doses (10, 25, 65 and 100 pmol) of senktide in spontaneously hypertensive rats. Areas under the curves (AUC) were measured for a period of 0–1 h (open columns for VTA and solid columns for i.c.v.). Values represent the mean ± SEM of six rats per injection site. Significant difference from aCSF values (*) or i.c.v. (†) is indicated by **††P < 0.01; ***†††P < 0.001.
When senktide (10–100 pmol) was injected directly into the VTA of SHR, it caused larger increases of MAP and HR than when injected i.c.v. (Figure 6B). These effects were, however, not dose-dependent and reached maximal values at 25 pmol for both parameters. The initial depressor response to senktide contributes to the smaller AUC for the MAP response at 65 pmol and may explain the absence of a dose-dependent cardiovascular effect.
Effect of the NK3R antagonist, R-820, on the cardiovascular response to senktide in the VTA
The increase in MAP and HR induced by senktide (25 pmol) injected into the VTA was completely abolished by the prior administration into the VTA of the NK3R antagonist R-820 (500 pmol, 1 h earlier) in SHR (Figure 7). The cardiovascular response to senktide was fully recovered 24 h later in the absence of R-820 (data not shown). When a microinjection of senktide (25 pmol, n = 6) was made outside the VTA, no cardiovascular effect was observed. Also, no changes in blood pressure occurred after microinjection of R-820 (500 pmol, n = 8) outside the VTA (data not shown).
Figure 7.
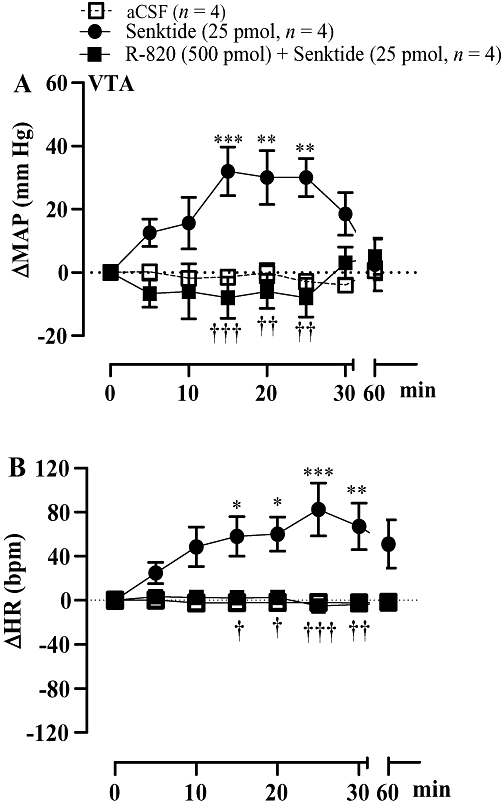
Time-course effects on changes in mean arterial blood pressure (ΔMAP) and heart rate (ΔHR) produced by senktide (25 pmol) injected into the ventral tegmental area (VTA) prior to (first day) and 1 h after VTA injection of R-820 (second day). Each point represents the mean ± SEM of four rats. Significant difference from aCSF (*) or senktide (†) values is indicated by *†P < 0.05; **††P < 0.01; ***†††P < 0.001.
Effects of dopamine receptor antagonists on the pressor response induced by i.c.v. senktide
The dopamine D1R antagonist SCH23390 (0.2 mg kg−1, i.v., 30 min earlier) blocked the pressor effect mediated by i.c.v. senktide (65 pmol). In contrast, the D2R antagonist raclopride (0.16 mg kg−1, i.v., 30 min previously) had a marginal effect on the pressor response induced by senktide (Figure 8A,C). The same treatment with haloperidol (10 mg kg−1, s.c., 30 min previously) abolished the pressor effect induced by senktide (65 pmol) injected i.c.v. in SHR (Figure 8B,C). The MAP response to senktide was completely recovered when it was re-injected alone 24 h after treatment with the dopamine antagonists (data not shown).
Figure 8.
Time-course effects on changes in mean arterial blood pressure (ΔMAP) produced by i.c.v. senktide 65 pmol (baseline: 161 ± 5 mmHg) prior to and after treatment with (A) the D1R antagonist SCH23390 (0.2 mg kg−1 i.v.) and the D2R antagonist raclopride (0.16 mg kg−1 i.v.) (baseline: 167 ± 4 mm Hg); and (B) the non-selective D2R antagonist haloperidol (10 mg kg−1 s.c.) (baseline: 164 ± 2.4 mm Hg) in spontaneously hypertensive rats. In (C), the areas under the curves (AUC) measured for a period of 0–1 h are shown. Values represent the mean ± SEM of n rats. Significant difference from aCSF (*) or senktide (†) values is indicated by *†P < 0.05; **††P < 0.01; ***†††P < 0.001.
At the dose used, the systemic administration of raclopride (0.16 mg kg−1, i.v.) caused a transient increase in MAP, which was significant in comparison with saline between 5 and 15 min post-injection. SCH23390 (0.2 mg kg−1, i.v.) had no significant effect on MAP during the first hour post-injection. Conversely, haloperidol (10 mg kg−1, s.c.) had an anti-hypertensive effect, which was significant from 1 h up to 6 h post-injection in comparison with saline (Figure 9). The maximal decrease in MAP induced by haloperidol at 3 h (−33 ± 5.5 mm Hg, P < 0.001) was back to hypertensive levels 8 h later (184.1 ± 3.8 mm Hg).
Figure 9.
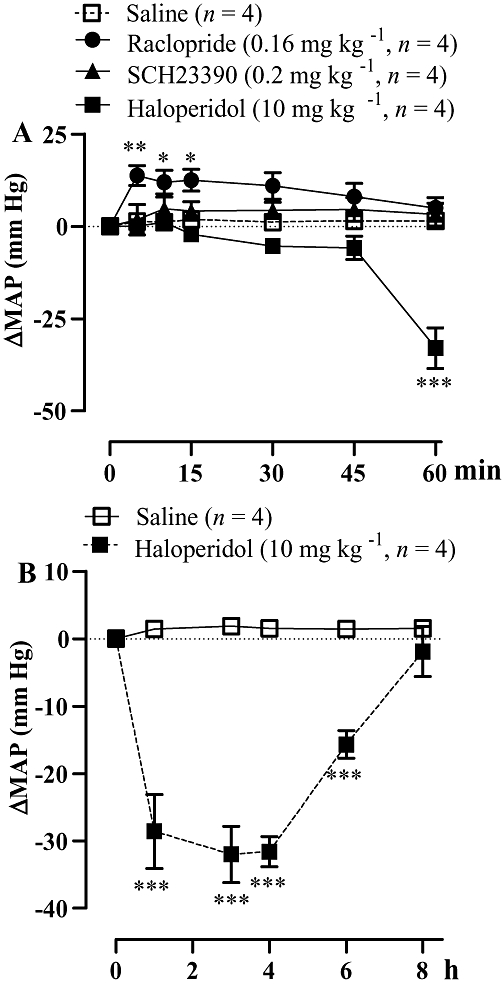
Time-course effects on changes in mean arterial blood pressure (ΔMAP) produced by treatments with the D1R antagonist SCH23390 (0.2 mg kg−1 i.v.), D2R antagonist raclopride (0.16 mg kg−1 i.v.) and the non-selective D2R antagonist haloperidol (10 mg kg−1 s.c.) in spontaneously hypertensive rats. Different time scales were used: in (A) (min), and (B) (h).Values represent the mean ± SEM of four rats. Significant difference from saline is indicated by *P < 0.05; **P < 0.01; ***P < 0.001.
Interaction between i.c.v. senktide and VTA SB222200 in SHR
The pressor response evoked by i.c.v. senktide (65 pmol) was blocked when SB222200 (500 pmol) was injected into the ipsilateral VTA, 1 h prior to a second injection of i.c.v. senktide (Figure 10A,E). In contrast, when SB222200 was injected into the VTA under the same conditions, the pressor response to senktide injected i.c.v. on the contralateral side was little affected (Figure 10B,F). Moreover, the anti-hypertensive effect, which occurred from 1 to 5 h after VTA injection of SB222200 (500 pmol), was blocked when senktide was injected i.c.v. on the ipsilateral side (Figure 10C,E), yet it was only slightly reduced when senktide was injected i.c.v. on the contralateral side (Figure 10D,F). This suggests that i.c.v. senktide diffused chiefly to the ipsilateral VTA to cause its pressor effect and to prevent the anti-hypertensive effect of SB222200. This also suggests that microinjected SB222200 into the VTA had limited diffusion to the contralateral VTA.
Figure 10.
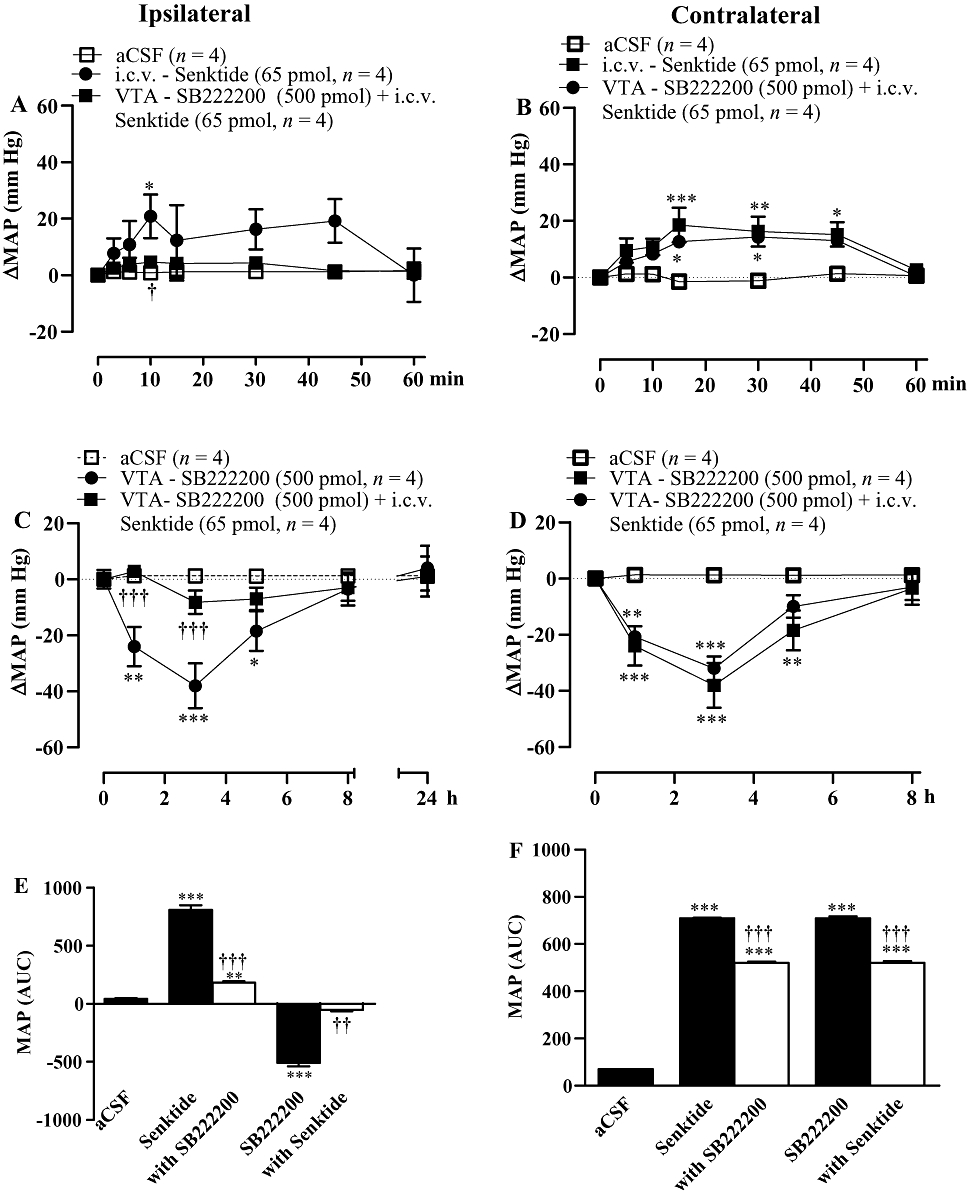
Time-course effects on changes in mean arterial blood pressure (ΔMAP) produced by senktide injected i.c.v. on the (A) ipsilateral and (B) contralateral sides before and after SB222200 (500 pmol) injected into the ventral tegmental area (VTA). The anti-hypertensive effect elicited by the VTA injection of SB222200 (500 pmol) was measured before and after i.c.v. injection of senktide (65 pmol) on the ipsilateral (C) and contralateral (D) side in spontaneously hypertensive rats. Areas under the curves (AUC) for MAP are shown in (E and F). Values represent the mean ± SEM of n rats. Significant difference from aCSF (*) and senktide alone or SB222200 alone (†) is indicated by *†P < 0.05; **††P < 0.01; ***†††P < 0.001.
Effect of ipsilateral and contralateral lesions of the VTA with IBO on the cardiovascular response to senktide and R-820
The pressor response induced by i.c.v. senktide (65 pmol) in sham-operated SHR was absent in rats subjected to ipsilateral lesion of the VTA with IBO (Figure 11A,E). One day after the senktide experiment, R-820 (500 pmol) was given i.c.v. to both groups of rats. Only the sham-operated rats displayed the typical anti-hypertensive response to R-820 (Figure 11C,E). Conversely, the pressor response induced by i.c.v. senktide (65 pmol) in sham-operated SHR was similar to that observed in rats subjected to contralatereal lesion of the VTA with IBO (Figure 11B,F). One day after the senktide experiment, the anti-hypertensive effect of i.c.v. R-820 (500 pmol) was very little affected by the contralateral lesion of the VTA (Figure 11D,F).
Figure 11.
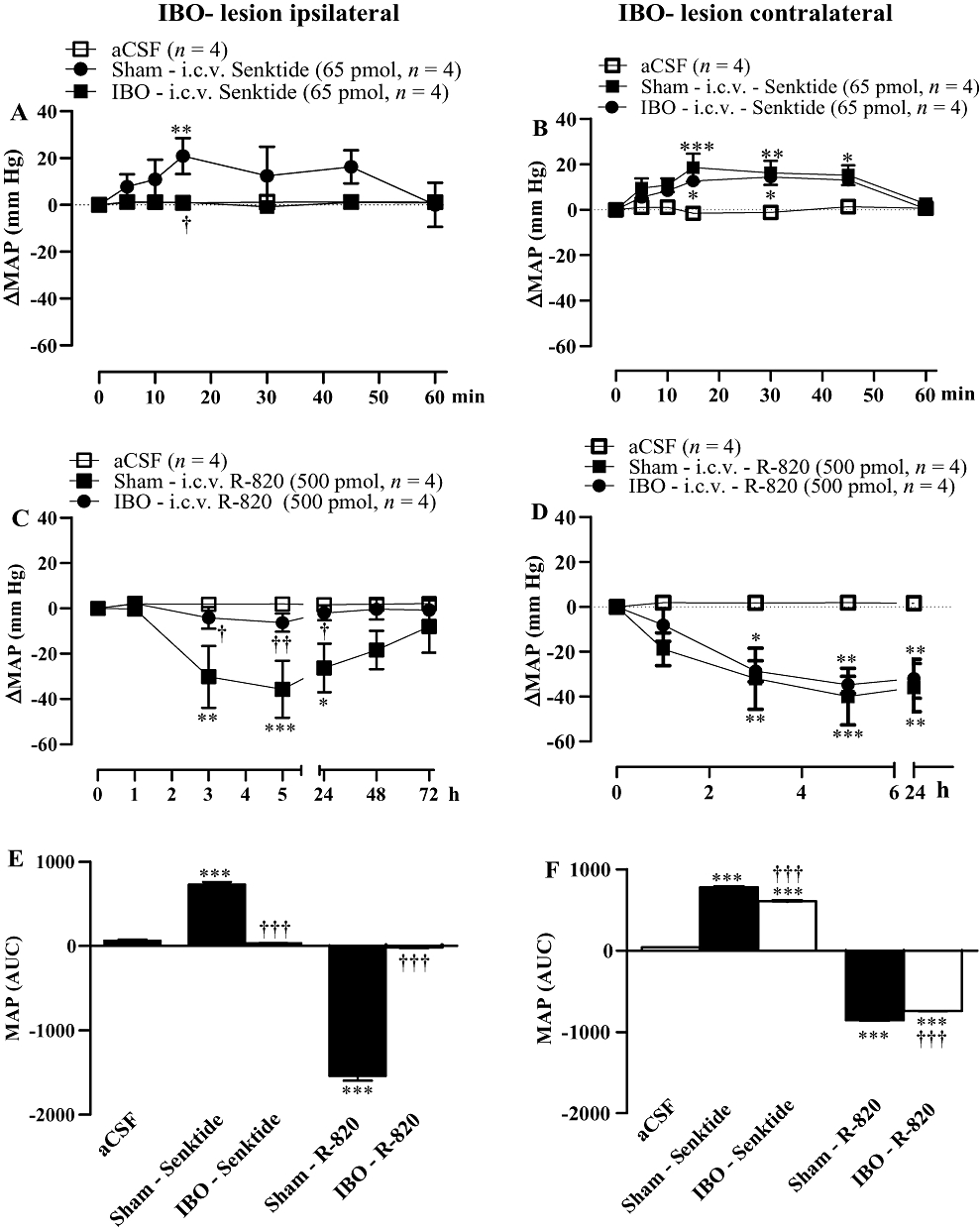
Time-course effects on changes in mean arterial blood pressure (ΔMAP) produced by i.c.v. injected 65 pmol senktide (A, B) or 500 pmol R-820 (C, D) in spontaneously hypertensive rats which underwent an ipsilateral (A,C) and contralateral (B,D) lesion of the ventral tegmental area with ibotenic acid (IBO), 5 days earlier or a sham-operation. Areas under the curves (AUC) for MAP are shown in (E,F). Each point represents the mean ± SEM of n rats. Significant difference from aCSF (*) or sham-operated rats (†) is indicated by *†P < 0.05; **††P < 0.01; ***†††P < 0.001.
Discussion
This study extends our earlier work showing that tachykinin NK3R antagonists exert dose-dependent and reversible anti-hypertensive effects when injected intracerebrally in SHR (Lessard et al., 2004). The present results demonstrate that this anti-hypertensive effect is mediated by the VTA and the mesocorticolimbic dopaminergic pathway. This statement is based on four findings: (i) the anti-hypertensive effect of NK3R antagonists injected i.c.v. was reproduced with a faster onset when drugs were injected directly into the VTA; (ii) the cardiovascular responses elicited by i.c.v. or VTA administration of NK3R agonist and antagonists were blocked by dopamine receptor antagonists; (iii) the i.c.v. pressor effect of the NK3R agonist was prevented following the inhibition of NK3R in the ipsilateral VTA, and vice versa, the anti-hypertensive effect of SB222200 injected into the VTA was blocked by an ipsilateral i.c.v injection of senktide; and (iv) the chemical destruction of the ipsilateral VTA with IBO eliminated completely the cardiovascular response induced by i.c.v. senktide and R-820. Hence, these findings suggest that the VTA and its dopaminergic projections in the brain are of primary importance in the cardiovascular effects associated with i.c.v. injections of an NK3R agonist and antagonist.
I.c.v. injection of senktide increases HR and blood pressure in normotensive rats (Cellier et al., 1997) and guinea pigs (Roccon et al., 1996). This cardiovascular response is blocked by the prior i.c.v. injection of the NK3R antagonist R-820 in normotensive rats (Cellier et al., 1997) and has been ascribed to the activation of the sympatho-adrenal system (Nagashima et al., 1989; Roccon et al., 1996). The cardiovascular response to senktide in the VTA, also blocked by R-820 (present study), is mediated by the sympathetic nervous system and the subsequent increase of cardiac output, as it has been shown to be abolished by i.v. treatment with atenolol, a β1-adrenoceptor antagonist (Deschamps and Couture, 2005). The latter study showed that the cardiovascular response to senktide in the VTA is similar to that caused by glutamic acid, which is consistent with neuronal cell activation. Moreover, the cardiovascular response to a central injection of senktide in SHR is probably mediated by DA release because it was abolished after treatment with SCH23390 and haloperidol. The lack of effect of raclopride suggests the involvement of D1R rather than D2R, as reported previously in normotensive rats (Deschamps and Couture, 2005). This is congruent with the release of DA in the nucleus accumbens following the activation of NK3R by senktide in the VTA (Spooren et al., 2005) and with the localization of NK3R on dopaminergic neurones in the VTA (Chen et al., 1998; Lessard et al., 2007; 2009;).
Our results suggest that tonic activation of NK3R in the VTA enhances the release of DA in the mesocorticolimbic system and contributes to high BP in SHR. This hypothesis is supported by the normalization of high BP induced by blockade of NK3R in the VTA through a mechanism sensitive to DA inhibition. The persistent anti-hypertensive effect induced by the 5 day i.c.v. administration of R-820 suggests that NK3R is under constant and chronic tonic activation in SHR. In addition, this experiment also substantiates the use of centrally acting NK3R antagonists for the treatment of hypertension in this model. As the anti-hypertensive effect of NK3R antagonists was prevented by raclopride but much less by SCH23390, it is concluded that this effect is chiefly mediated by D2R. The suggestion of a greater basal release of tachykinins in the mesocorticolimbic system of SHR is consistent with the existence of endogenous tachykinins in the VTA (Kalivas et al., 1985; Warden and Young, 1988; Tamiya et al., 1990; Lu et al., 1998) and the increased neurokinin B-like immunoreactive contents in the brain of SHR, the putative NK3R endogenous ligand (Nagashima et al., 1989). Because D1R and D2R are up-regulated in the nucleus accumbens in SHR (Kirouac and Ganguly, 1993; Vaughan et al., 1999), it is tempting to speculate that the tonic activation of NK3R by endogenous tachykinins in the VTA is facilitated by a hyperactive dopaminergic mesolimbic system. Reciprocally, tonic activation of NK3R may contribute to the hyperactivity of the VTA dopaminergic neuronal pathway and thereby in the maintenance of hypertension in SHR. The affinity and densities of specific NK3R binding sites measured in the VTA and other midbrain nuclei are, however, not significantly different between SHR and WKY (Lessard et al., 2003).
As i.c.v. injection of NK3R antagonists has no significant effect on resting BP in normotensive rats, it was concluded that brain NK3R did not exert a tonic autonomic control of BP in normotensive rats (Couture et al., 1995; Cellier et al., 1997; Lessard et al., 2004; Deschamps and Couture, 2005). Nevertheless, the mesolimbic dopaminergic system is involved in the diurnal regulation of BP and HR (Sei et al., 1999; Sakata et al., 2002), and therefore, the VTA may represent an important site of action for tachykinins in cardiovascular regulation in normotensive subjects.
Whereas SB222200 and R-820 induced tachycardia when injected into the VTA, both antagonists induced a small bradycardia at the peak of the anti-hypertensive response after i.c.v. injection. The occurrence of tachycardia in response to a fall in BP suggests that the inhibition of NK3R in the VTA did not interfere with the activation of baroreceptors, in contrast to the i.c.v. administration of NK3R antagonists, and further suggests that the inhibition of the cardiac output or baroreflex activity is unlikely to be the mechanism of the anti-hypertensive effect of these antagonists. Although the increase in BP and HR induced by i.c.v and VTA-injected senktide has been ascribed to the activation of the sympathetic nervous system (Roccon et al., 1996; Deschamps and Couture, 2005), the decrease in BP induced by i.c.v. injected SB222200 is not associated with changes in plasma catecholamine levels in SHR (Lessard et al., 2004). However, the possibility that central tachykinin NK3R antagonists may exert an inhibitory action on neuronal sympathetic vasomotor tone cannot be excluded.
The dissimilar time-course of the anti-hypertensive effect of R-820 and SB222200 was also observed in our previous study (Lessard et al., 2004) and may be explained by the different physico-chemical features of these two compounds. SB222200, a much more lipophilic molecule than R-820, can diffuse more easily into the brain after i.c.v. injection. This could explain why SB222200 displays a quicker but less prolonged effect than R-820. The peripheral administration of these antagonists at 500 pmol in SHR had no effect on BP, supporting a central site of action (Lessard et al., 2004) that has been identified in the present study as the VTA.
The anti-hypertensive effect of s.c. administered haloperidol in SHR was not shared by selective blockade of D1R and D2R, which caused either no effect or a transient pressor response. The non-selectivity of haloperidol versus SCH23390 and raclopride for DA receptors, and the putative inhibition of non-DA receptors can account for the pronounced anti-hypertensive response of haloperidol. Although data of the present study obtained with haloperidol support the conclusion drawn with the selective DA antagonist SCH23390 on the cardiovascular effect of senktide, they must be interpreted with caution. The reduction in MAP with haloperidol has also been observed in angiotensin II-hypertensive rats, while the same treatment had no effect in normotensive rats (De Brito Gariepy et al., 2010). In addition, it has been suggested that haloperidol could be used to treat hypertensive crises caused by high-dose amphetamine or methamphetamine abuse (Angrist et al., 2001).
Conclusion
This neuropharmacological study suggests that tachykinin NK3R located in the VTA is subjected to persistent tonic activation, which increases midbrain dopaminergic transmission and thereby contributes to hypertension in SHR. Hence, these receptors may represent a therapeutic target in the treatment of arterial hypertension. Since NK3R antagonists have been proposed as putative antipsychotics in the treatment of schizophrenia (Spooren et al., 2005; Meltzer and Prus, 2006), understanding the functional interaction between NK3R and the dopaminergic system in the central autonomic control of BP is of prime importance.
Acknowledgments
This work was supported by a Grant-in-aid from the Canadian Institutes of Health Research (MOP-79471). H.B.G. held a Studentship from the National Council of Technological and Scientific Development (CNPq) (Proc. 201428/2003-2) Brazil. We are grateful to Ms Paula Jansen Santana for the Artwork of Figure 1.
Glossary
Abbreviations
- aCSF
artificial cerebrospinal fluid
- BP
blood pressure
- DA
dopamine
- DMSO
dimethylsulphoxide
- HR
heart rate
- MAP
mean arterial blood pressure
- NKA
neurokinin A
- NKB
neurokinin B
- NK1R
tachykinin NK1 receptor
- NK2R
tachykinin NK2 receptor
- NK3R
tachykinin NK3 receptor
- SHR
spontaneously hypertensive rats
- SP
substance P
- WKY
Wistar Kyoto
- VTA
ventral tegmental area
Conflicts of interest
None.
Supporting Information
Teaching Materials; Figs 1–11 as PowerPoint slide.
References
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 4th edn. Br J Pharmacol. 2009;158(Suppl. 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amenta F, Ricci A, Rossodivita I, Avola R, Tayebati SK. The dopaminergic system in hypertension. Clin Exp Hypertens. 2001;23:15–24. doi: 10.1081/ceh-100001193. [DOI] [PubMed] [Google Scholar]
- Angrist B, Sanfilipo M, Wolkin A. Cardiovascular effects of 0.5 milligrams per kilogram oral d-amphetamine and possible attenuation by haloperidol. Clin Neuropharmacol. 2001;24:139–144. doi: 10.1097/00002826-200105000-00004. [DOI] [PubMed] [Google Scholar]
- Cador M, Rivet JM, Kelley AE, Le Moal M, Stinus L. Substance P, neurotensin and enkephalin injections into the ventral tegmental area: comparative study on dopamine turnover in several forebrain structures. Brain Res. 1989;486:357–363. doi: 10.1016/0006-8993(89)90523-4. [DOI] [PubMed] [Google Scholar]
- Carson R. Histotechnology: A Self-Instructional Text. 1st edn. Chicago: ASCP Press; 1992. [Google Scholar]
- Cellier E, Barbot L, Regoli D, Couture R. Cardiovascular and behavioural effects of intracerebroventricularly administered tachykinin NK3 receptor antagonists in the conscious rat. Br J Pharmacol. 1997;122:643–654. doi: 10.1038/sj.bjp.0701435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LW, Guan ZL, Ding YQ. Mesencephalic dopaminergic neurons expressing neuromedin K receptor (NK3): a double immunocytochemical study in the rat. Brain Res. 1998;780:148–152. [PubMed] [Google Scholar]
- Cornish JL, Van den Buuse M. Stimulation of the rat mesolimbic dopaminergic system produces a pressor response which is mediated by dopamine D1 and D2 receptor activation and the release of vasopressin. Brain Res. 1995;701:28–38. doi: 10.1016/0006-8993(95)00967-x. [DOI] [PubMed] [Google Scholar]
- Couture R, Picard P, Poulat P, Prat A. Characterization of the tachykinin receptors involved in spinal and supraspinal cardiovascular regulation. Can J Physiol Pharmacol. 1995;73:892–902. doi: 10.1139/y95-123. [DOI] [PubMed] [Google Scholar]
- Cuello AC, Emson PC, Paxinos G, Jessell T. Substance P containing and cholinergic projections from the habenula. Brain Res. 1978;149:413–429. doi: 10.1016/0006-8993(78)90484-5. [DOI] [PubMed] [Google Scholar]
- Dam T-V, Escher E, Quirion R. Visualization of neurokinin-3 receptor sites in rat brain using the highly selective ligand [3H] senktide. Brain Res. 1990;506:175–179. doi: 10.1016/0006-8993(90)91218-6. [DOI] [PubMed] [Google Scholar]
- De Brito Gariepy H, Carayon P, Ferrari B, Couture R. Contribution of the central dopaminergic system in the anti-hypertensive effect of kinin B1 receptor antagonists in two rat models of hypertension. Neuropeptides. 2010;44:191–198. doi: 10.1016/j.npep.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Deschamps K, Couture R. The ventral tegmental area as a putative target for tachykinins in cardiovascular regulation. Br J Pharmacol. 2005;145:712–727. doi: 10.1038/sj.bjp.0706249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutch AY, Maggio JE, Bannon MJ, Kalivas PW, Tam SY, Goldstein M, et al. Substance K and substance P differentially modulate mesolimbic and mesocortical systems. Peptides. 1985;6(Suppl. 2):113–122. doi: 10.1016/0196-9781(85)90143-3. [DOI] [PubMed] [Google Scholar]
- Ding YQ, Shigemoto R, Takada M, Ohishi H, Nakanishi S, Mizuno N. Localization of the neuromedin K receptor (NK3) in the central nervous system of the rat. J Comp Neurol. 1996;364:290–310. doi: 10.1002/(SICI)1096-9861(19960108)364:2<290::AID-CNE8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Eison AS, Eison MS, Iversen SD. The behavioural effects of a novel substance P analogue following infusion into the ventral tegmental area or substantia nigra of rat brain. Brain Res. 1982;238:137–152. doi: 10.1016/0006-8993(82)90777-6. [DOI] [PubMed] [Google Scholar]
- Elliott PJ, Iversen SD. Behavioural effects of tachykinins and related peptides. Brain Res. 1986;381:68–76. doi: 10.1016/0006-8993(86)90691-8. [DOI] [PubMed] [Google Scholar]
- Elliott PJ, Alpert JE, Bannon MJ, Iversen SD. Selective activation of mesolimbic and mesocortical dopamine metabolism in rat brain by infusion of a stable substance P analogue into the ventral tegmental area. Brain Res. 1986;363:145–147. doi: 10.1016/0006-8993(86)90667-0. [DOI] [PubMed] [Google Scholar]
- Gioanni Y, Thierry AM, Glowinski J, Tassin JP. Alpha1-adrenergic, D1, and D2 receptors interactions in the prefrontal cortex: implications for the modality of action of different types of neuroleptics. Synapse. 1998;30:362–370. doi: 10.1002/(SICI)1098-2396(199812)30:4<362::AID-SYN3>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Guldin WO, Markowitsch HJ. No detectable remote lesions following massive intrastriatal injections of ibotenic acid. Brain Res. 1981;225:446–451. doi: 10.1016/0006-8993(81)90852-0. [DOI] [PubMed] [Google Scholar]
- Guldin WO, Markowitsch HJ. Epidural kainate, but not ibotenate, produces lesions in local and distant regions of the brain. A comparison of the intracerebral actions of kainic acid and ibotenic acid. J Neurosci Methods. 1982;5:83–93. doi: 10.1016/0165-0270(82)90055-3. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Deutch AY, Maggio JE, Mantyh PW, Roth RH. Substance K and substance P in the ventral tegmental area. Neurosci Lett. 1985;57:241–246. doi: 10.1016/0304-3940(85)90498-7. [DOI] [PubMed] [Google Scholar]
- Kirouac GJ, Ciriello J. Cardiovascular depressor responses to stimulation of substantia nigra and ventral tegmental area. Am J Physiol Heart Circ Physiol. 1997;273:H2549–H2557. doi: 10.1152/ajpheart.1997.273.6.H2549. [DOI] [PubMed] [Google Scholar]
- Kirouac GJ, Ganguly PK. Up-regulation of dopamine receptors in the brain of the spontaneously hypertensive rat: an autoradiographic analysis. Neuroscience. 1993;52:135–141. doi: 10.1016/0306-4522(93)90188-l. [DOI] [PubMed] [Google Scholar]
- Langlois X, Wintmolders C, Te Riele P, Leysen JE, Jurzak M. Detailed distribution of neurokinin-3 receptors in the rat, guinea pig and gerbil brain: a comparative autoradiographic study. Neuropharmacology. 2001;40:242–253. doi: 10.1016/s0028-3908(00)00149-0. [DOI] [PubMed] [Google Scholar]
- Le Moal M, Simon H. Mesocorticolimbic dopaminergic network: functional and regulatory roles. Physiol Rev. 1991;71:155–234. doi: 10.1152/physrev.1991.71.1.155. [DOI] [PubMed] [Google Scholar]
- Lessard A, Campos MM, Neugebauer W, Couture R. Implication of nigral tachykinin NK3 receptors in the maintenance of hypertension in spontaneously hypertensive rats: a pharmacologic and autoradiographic study. Br J Pharmacol. 2003;138:554–563. doi: 10.1038/sj.bjp.0705042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessard A, Laurin M, Yamaguchi N, Couture R. Central anti-hypertensive effect of tachykinin NK3 receptor antagonists in rat. Eur J Pharmacol. 2004;486:75–83. doi: 10.1016/j.ejphar.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Lessard A, Grady EF, Bunnett NW, Pickel VM. Predominant surface distribution of neurokinin-3 receptors in non-dopaminergic dendrites in the rat substantia nigra and ventral tegmental area. Neuroscience. 2007;144:1393–1408. doi: 10.1016/j.neuroscience.2006.10.058. [DOI] [PubMed] [Google Scholar]
- Lessard A, Savard M, Gobeil F, Jr, Pierce JP, Pickel VM. The neurokinin-3 (NK3) and the neurokinin-1 (NK1) receptors are differentially targeted to mesocortical and mesolimbic projection neurons and to neuronal nuclei in the rat ventral tegmental area. Synapse. 2009;63:484–501. doi: 10.1002/syn.20627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XY, Ghasemzadeh MB, Kalivas PW. Expression of D1 receptor, D2 receptor, substance P and enkephalin messenger RNAs in the neurons projecting from the nucleus accumbens. Neuroscience. 1998;82:767–780. doi: 10.1016/s0306-4522(97)00327-8. [DOI] [PubMed] [Google Scholar]
- Marco N, Thirion A, Mons G, Bougault I, Le Fur G, Soubrie P, et al. Activation of dopaminergic and cholinergic neurotransmission by tachykinin NK3 receptor stimulation: an in vivo microdialysis approach in guinea pig. Neuropeptides. 1998;32:481–488. doi: 10.1016/s0143-4179(98)90075-0. [DOI] [PubMed] [Google Scholar]
- Meltzer H, Prus A. NK3 receptor antagonists for the treatment of schizophrenia. Drug Discov Today Ther Strateg. 2006;3:555–560. [Google Scholar]
- Millan MJ, Newman-Tancredi A, Brocco M, Gobert A, Lejeune F, Audinot V, et al. S 18126 ([2-[4-(2, 3-dihydrobenzo[1, 4]dioxin-6-yl)piperazin-1-yl methyl]indan-2-yl]), a potent, selective and competitive antagonist at dopamine D4 receptors: an in vitro and in vivo comparison with L 745, 870 (3-(4-[4-chlorophenyl]piperazin-1-yl)methyl-1H-pyrrolo[2, 3b]pyridine) and raclopride. J Pharmacol Exp Ther. 1998;287:167–186. [PubMed] [Google Scholar]
- Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10:79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- Nagashima A, Takano Y, Tateishi K, Matsuoka Y, Hamaoka T, Kamiya H. Central pressor actions of neurokinin B: increases in neurokinin B contents in discrete nuclei in spontaneously hypertensive rats. Brain Res. 1989;499:198–203. doi: 10.1016/0006-8993(89)91154-2. [DOI] [PubMed] [Google Scholar]
- Nsimba SED, Kelly JP, Leonard BE. Effect of acute and chronic haloperidol administration and apomorphine challenge on the behavioural parameters in rats. Indian J Pharmacol. 1997;29:15–19. [Google Scholar]
- Overton P, Elliott PJ, Hagan RM, Clark D. Neurokinin agonists differentially affect A9 and A10 dopamine cells in the rat. Eur J Pharmacol. 1992;213:165–166. doi: 10.1016/0014-2999(92)90251-x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Regoli D, Boudon A, Fauchere JL. Receptors and antagonists for substance P and related peptides. Pharmacol Rev. 1994;46:551–599. [PubMed] [Google Scholar]
- Roccon A, Marchionni D, Nisato D. Study of SR 142801, a new potent non-peptide NK3 receptor antagonist on cardiovascular responses in conscious guinea-pig. Br J Pharmacol. 1996;118:1095–1102. doi: 10.1111/j.1476-5381.1996.tb15511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell VA. Dopamine hypofunction possibly results from a defect in glutamate-stimulated release of dopamine in the nucleus accumbens shell of a rat model for attention deficit hyperactivity disorder-the spontaneously hypertensive rat. Neurosci Biobehav Rev. 2003;27:671–682. doi: 10.1016/j.neubiorev.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Sakata M, Sei H, Toida K, Fujihara H, Urushihara R, Morita Y. Mesolimbic dopaminergic system is involved in diurnal blood pressure regulation. Brain Res. 2002;928:194–201. doi: 10.1016/s0006-8993(01)03402-3. [DOI] [PubMed] [Google Scholar]
- Sarau HM, Griswold DE, Bush B, Potts W, Sandhu P, Lundberg D, et al. Nonpeptide tachykinin receptor antagonists. II. Pharmacological and pharmacokinetic profile of SB-222200, a central nervous system penetrant, potent and selective NK3 receptor antagonist. J Pharmacol Exp Ther. 2000;295:373–381. [PubMed] [Google Scholar]
- Seabrook GR, Patel S, Hutson PH, Ragan CI, Bristow LJ. Functional relevance of dopamine receptors: Further studies are required to determine the physiological role and therapeutic significance of dopamine D3 receptors. Trends Pharmacol Sci. 1995;16:406–408. doi: 10.1016/s0165-6147(00)89088-4. [DOI] [PubMed] [Google Scholar]
- Sei H, Ikemoto K, Arai R, Morita Y. Injection of 6-hydroxydopamine into the ventral tegmental area suppresses the increase in arterial pressure during REM sleep in the rat. Sleep Res. 1999;2:1–6. [PubMed] [Google Scholar]
- Spooren W, Riemer C, Meltzer H. NK3 receptor antagonists: the next generation of antipsychotics? Nat Rev Drug Discov. 2005;4:967–975. doi: 10.1038/nrd1905. [DOI] [PubMed] [Google Scholar]
- Stoessl AJ, Hill DR. Autoradiographic visualization of NK-3 tachykinin binding sites in the rat brain, utilizing [3H]senktide. Brain Res. 1990;534:1–7. doi: 10.1016/0006-8993(90)90105-k. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Flodman P, Kennedy J, Spence MA, Moyziz R, Schuck S, et al. Dopamine genes and ADHD. Neurosci Biobehav Rev. 2000;24:21–25. doi: 10.1016/s0149-7634(99)00062-7. [DOI] [PubMed] [Google Scholar]
- Tamiya R, Hanada M, Kawai Y, Inagaki S, Takagi H. Substance P afferents have synaptic contacts with dopaminergic neurons in the ventral tegmental area of the rat. Neurosci Lett. 1990;110:11–15. doi: 10.1016/0304-3940(90)90779-9. [DOI] [PubMed] [Google Scholar]
- Van den Buuse M. Pressor responses to brain dopaminergic stimulation. Clin Exp Pharmacol Physiol. 1997;24:764–769. doi: 10.1111/j.1440-1681.1997.tb02129.x. [DOI] [PubMed] [Google Scholar]
- Van den Buuse M. Role of the mesolimbic dopamine system in cardiovascular homeostasis. Stimulation of the ventral tegmental area modulates the effect of vasopressin on blood pressure in conscious rats. Clin Exp Pharmacol Physiol. 1998;25:661–668. doi: 10.1111/j.1440-1681.1998.tb02273.x. [DOI] [PubMed] [Google Scholar]
- Van den Buuse M, Catanzariti R. Stimulation of the ventral tegmental area enhances the effect of vasopressin on blood pressure in conscious rats. Br J Pharmacol. 2000;129:29–36. doi: 10.1038/sj.bjp.0702982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CE, Van den Buuse M, Roland BL. Brain dopamine D2 receptor mRNA levels are elevated in young spontaneously hypertensive rats. Neurosci Res. 1999;34:199–205. doi: 10.1016/s0168-0102(99)00037-1. [DOI] [PubMed] [Google Scholar]
- Warden MK, Young WS., 3rd Distribution of cells containing mRNAs encoding substance P and neurokinin B in the rat central nervous system. J Comp Neurol. 1988;272:90–113. doi: 10.1002/cne.902720107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


