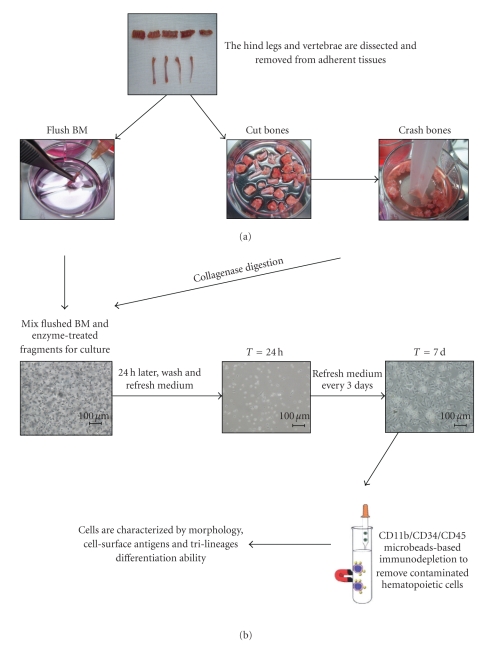
Figure 1.
Flow chart for our modified harvest and expansion protocol. (a) Mice were sacrificed by cervical dislocation. The hind legs and vertebrae were dissected and carefully removed from adherent tissues. The BM was collected by flushing out the content of femurs and tibias with RPMI 1640. Next, the femurs, tibias, and vertebrae were cutted into small pieces and crushed gently. Next, the bone fragments were incubated with 0.25% Collagenase A solution in 37°C water bath for 30 minutes. (b) Collagenase-treated bone fragments were mixed with the previously flushed BM cells, filtered through 70-μm nylon mesh filter and cultured at 1 × 106 cells/cm2. At 24 h after initial culture, the nonadherent cells were washed away. After about 7–10 days, when primary cultures became nearly confluent, the cells were trypsinized, followed by a CD11b/CD34/CD45 negative immunodepletion in order to remove contaminated hematopoietic cells. The immunodepleted cells were cultivated and characterized at the level of their morphology, immunophenotype, and differentiation potentials. Scale bar = 100 μm.