Summary Statement
Human milk is a viable source of DNA for pharmacogenetic studies, although unpasteurized samples demonstrate stronger amplification.
Introduction
Studies consistently find beneficial effects of breastfeeding on overall maternal and infant health.(1) Breast-fed infants have a significantly lower incidence of respiratory infection, otitis media, diarrhea, and dehydration, as well as fewer signs and symptoms of atopic disease.(2-4) These health advantages underscore the importance of maintaining the breastfeeding relationship for mother and infant as long as possible.
Although many healthcare providers actively encourage breastfeeding for mothers and infants, there are clinical situations that require evidence-based information to inform breastfeeding management. One such situation is decision-making regarding the safety of continuing breastfeeding in the face of needed maternal medications.
The current evidence regarding medication and breastfeeding utilizes measurement of drug concentrations in human milk. While strides are being made in human milk pharmacology,(5) it is important to understand the potential role of genetic variability in drug disposition and clinical response- including the impact of genetic changes on drug concentrations in human milk. By identifying genetic factors that are associated with variability in drug concentrations in human milk, we may be able to prospectively provide better guidance to breastfeeding mothers needing to take medications. As in other areas of medical science, pharmacogenetics may be able to advance the human milk science regarding medication use and breastfeeding. In order to perform pharmacogenetic studies, researchers need to obtain deoxyribonucleic acid (DNA) from a human biologic sample. Banked milk samples may help unlock some of the reasons for differences in response, toxicity, or milk drug concentrations if DNA could be extracted sufficiently from these specimens, making them useful for pharmacogenetic studies.
The purpose of this study was to determine whether or not DNA could be extracted from human milk in significant amounts to be clinically useful in pharmacogenetic studies. This approach may provide an explanation for inter-patient variability of drug expression into human milk and in exposure of the recipient infant. This is a first step toward integrating pharmacogenetics into human milk pharmacology and breastfeeding management.
Materials and Methods
This project was approved by the local Institutional Review Board in an exempt status. Milk samples were obtained from 37 anonymous lactating mothers who donated to a local milk bank. The samples contained both unpasteurized and pasteurized aliquots from each individual donor. All laboratory procedures were performed in accordance with standard best operating practices in the Pharmacogenetics Core Laboratory at Indiana University School of Medicine.
The 74 paired samples (pasteurized and unpasteurized) were divided into 1ml aliquots and placed into Eppendorf tubes. 200μl of whole human milk was removed from the 1ml aliquot and DNA was extracted. The Qiagen® blood mini kit (Qiagen Inc. USA, Valencia, CA) was used to extract the DNA using the manufacturer's instructions, with the exception of using milk rather than blood as the source. The DNA was then quantified with the Qubit Quantitation Platform (Invitrogen, Inc, Carlsbad, CA, USA). To determine the quality of the DNA, and its suitability for polymerase chain reaction (PCR) amplification, we used a multiplexed PCR amplification that amplifies 7 different size fragments of the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene as originally described by van Beers(6) (100, 200, 300, and 400 bp) and modified to include an additional 3 larger amplicons (500, 600, and 700 bp) by Sikora et al.(7) We used 20ng of DNA per reaction and ran the PCR for 35 cycles. By amplifying multiple different sized fragments of the GAPDH gene, this assay provides a measure of the quality of the DNA as determined by the length of DNA that can be successfully amplified. DNA amplicons were analyzed on the Agilent Bioanalyzer 2100.
Mean concentrations were compared using the Mann-Whitney U test for nonparametric data. Chi-square testing was utilized to compare the strength of the amplification of each sample, with the curves being categorized as “no amplification” if no DNA amplification peaks were seen, “weak amplification” if only small peaks were seen in a few of the key coding regions, or “strong amplification” if numerous distinguishable peaks were visualized. Representative chromatograms from each of these samples are shown in Figure 1.
Figure 1. Representative chromatograms of PCR analysis of milk DNA samples.

PCR products were analyzed on the Agilent Bioanalyzer 2100 DNA 1,000 chip. The chromatograms are representative of samples that showed no amplification (top), weak amplification (middle), or strong amplification (bottom). Y-axis is the fluorescence detection of the PCR product. X-axis is the size of the amplicon in nucleotides. The first and last peaks are the internal standard markers (15 and 1500 nucleotides).
Results
We were able to extract DNA from human milk in quantifiable amounts from all pasteurized and unpasteurized samples. The mean concentration of DNA in unpasteurized human milk was 2.6 ± 2.0 ng/μl, (range 1.7-12.5 ng/μg; 340-2500 total ng) compared to 2.0 ± 1.8 ng/μl (range 1.6-12.5 ng/μl, 320-2520 total ng) for pasteurized samples (p<0.001).
DNA was amplified from all unpasteurized specimens. However, we were not able to amplify the GAPDH gene from 16 pasteurized specimens (p<0.001). The distribution of the strength of amplification is shown in Figure 2. Unpasteurized specimens were associated with better amplification than pasteurized specimens, as indicated by the greater percentage with strong amplification and fewer with weak amplification (p<0.001).
Figure 2. Comparison of DNA amplification from unpasteurized and pasteurized banked human breast milk samples.
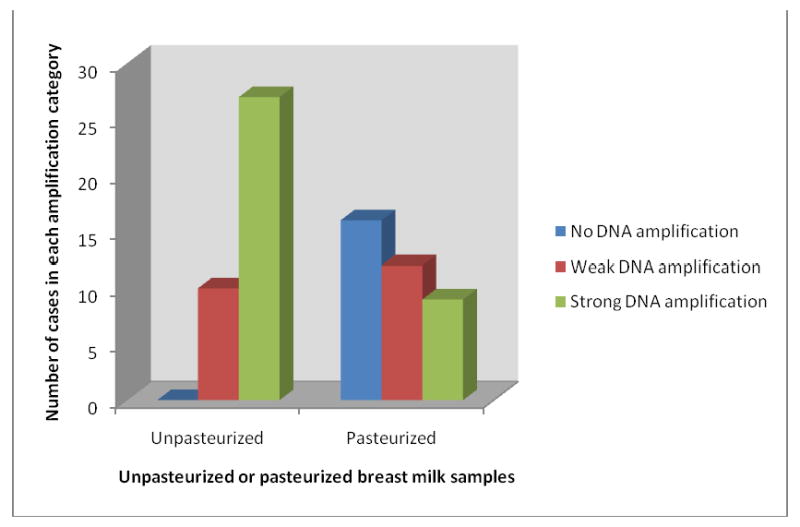
The quality of DNA amplification was determined by PCR DNA amplification at multiple key coding regions and analyzed by the Luminex machine. Comparison p<0.001.
Discussion
To our knowledge, this is the first report of extracting and amplifying DNA from paired pasteurized and unpasteurized donor human milk samples. These data indicate that DNA can be extracted in small but quantifiable amounts from human milk. Additionally, most of the samples, particularly the unpasteurized ones, were suitable for DNA amplification. DNA has been extracted from bovine milk in quantities between 11 and 1100 μg.(8) In the animal model, as in our model, milk may be a less reliable source of DNA than is blood because of the lower yield. The source of the DNA in the milk is likely a combination of mammary epithelial cells and infiltrating immune cells. Although we do not know the quantitative contribution from each source, it is likely not important for pharmacogenetic studies since the DNA sequence of the relevant genes should be the same in the two cell types.
Healthcare providers have concerns with administration of drugs to lactating and breastfeeding women, and also the consumption of the milk by the infants. However, there are chronic conditions, as well as conditions that develop in the peripartum period, that require drug therapy. These conditions include but are not limited to hypertension, mood disorders, and thyroid disorders. Many women take drugs as a part of a newly prescribed or existing pharmacological treatment while lactating and breastfeeding. Because these drugs can migrate to human milk, it is important to understand the response of the recipient infant to specific doses with regard to possible adverse neuro-developmental effects and toxicity.(9, 10) High inter-individual variability with regard to the amount of drug expressed in human milk, as well as the concentration of drug present in infant serum following breast feeding, has been reported for several medications.(9, 11-17) Most of these reports, however, are lacking pharmacogenetic information and thus may not contribute to the understanding of the variable drug exposure in these infants.
The presence of DNA in human milk is important in its utilization potential for pharmacogenetic studies. Human milk is collected and banked in clinical settings that include human milk banking as well as early human studies for drug development. Samples collected and banked for drug development are annotated with accurate medication dosage information, maternal and outcome information, and often drug pharmacokinetic information. While saliva and whole blood samples would yield more and possibly higher quality DNA from these subjects, those samples are not present in many human milk banks. Being able to extract and amplify DNA from these banked human milk specimens would allow researchers to factor in genetics as a possible explanation for differences seen in drug concentrations in human milk and resulting neonatal outcomes. Knowing the genetic status of the mother's metabolizing enzyme genes may elucidate why women taking the same dose of medication have different drug concentrations in their breast milk. Overlaying pharmacogenetics with the information in current banks could be used to advance human milk science and potentially yield useful clinical tests to aid in breastfeeding management for women who need to take medications while breastfeeding. It will be important to assure that the appropriate consent is obtained for the simultaneous analysis of drug and genetic parameters, although we do not expect they would be substantially different from similar studies serum pharmacogenetic studies.
The finding that many of the pasteurized samples contained measurable DNA that was not amplifiable indicates that pasteurization does not eliminate the presence of DNA from human milk; but it may affect the quality of the DNA present. This indicates that the pasteurized human milk samples maybe be less useful in milk-based pharmacogenetic studies. However, most samples banked for drug research are not pasteurized and may be useful for pharmacogenetic studies. Future studies will need to focus on the genotyping of specific candidate pharmacogenetic genes to determine if they can be amplified similar to the GAPDH.
These results show that DNA can be extracted from human milk and may hold promise for the use of unpasteurized human milk as a possible alternative to blood as a source of DNA for pharmacogenetic studies. Adding pharmacogenetics to current knowledge about medications in human milk and the dose response for recipient infants will provide evidence-based information for breastfeeding management. Healthcare provider encouragement of breastfeeding is known to increase duration leading to improved infant health outcomes.(18, 19) Human milk science and pharmacology are areas for pharmacogenetics to impact clinical practice as well as the lives of women and infants.
Acknowledgments
The authors thank the Indiana Mothers' Milk Bank for the donation of the human milk samples used in this project. This work was supported by grants: NIH-NICHD K23HD055305 (Haas), NIH-NIGMS 1R01GM088076 (Skaar), NIH-Pharmacogenetics Research Network 5U01GM061373 (Skaar), and the Indiana University-Purdue University- Indianapolis Signature Center Grant to PREGMED- The Indiana University Center for Pharmacogenetics and Therapeutics Research in Maternal and Child Health.
References
- 1.Ip S, et al. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess. 2007:1–186. Full Rep. [PMC free article] [PubMed] [Google Scholar]
- 2.Chandra RK. Prospective studies of the effect of breast feeding on incidence of infection and allergy. Acta Paediatr Scand. 1979;68:691–4. doi: 10.1111/j.1651-2227.1979.tb18439.x. [DOI] [PubMed] [Google Scholar]
- 3.Howie PW, Forsyth JS, Ogston SA, Clark A, Florey CD. Protective effect of breast feeding against infection. Bmj. 1990;300:11–6. doi: 10.1136/bmj.300.6716.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palti H, Mansbach I, Pridan H, Adler B, Palti Z. Episodes of illness in breast-fed and bottle-fed infants in Jerusalem. Isr J Med Sci. 1984;20:395–9. [PubMed] [Google Scholar]
- 5.Hale T. Medications and Mother's Milk. 12th. Hale Publishing, L.P.; Amarillo, TX: 2006. [Google Scholar]
- 6.van Beers EH, et al. A multiplex PCR predictor for aCGH success of FFPE samples. Br J Cancer. 2006;94:333–7. doi: 10.1038/sj.bjc.6602889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sikora M, Thibert JN, Salter J, Dowsett M, Johnson MD, Rae JM. High throughput SNP analysis from formalin-fixed, paraffin-embedded tumor tissues [abstract]. Proceedings of the 100th Annual Meeting of the American Association for Cancer Research; Denver, CO. 2009. Abstract #1642. [Google Scholar]
- 8.Lipkin E, Shalom A, Khatib H, Soller M, Friedmann A. Milk as a source of deoxyribonucleic acid and as a substrate for the polymerase chain reaction. J Dairy Sci. 1993;76:2025–32. doi: 10.3168/jds.S0022-0302(93)77536-0. [DOI] [PubMed] [Google Scholar]
- 9.Gentile S. The safety of newer antidepressants in pregnancy and breastfeeding. Drug Saf. 2005;28:137–52. doi: 10.2165/00002018-200528020-00005. [DOI] [PubMed] [Google Scholar]
- 10.Koren G, Cairns J, Chitayat D, Gaedigk A, Leeder SJ. Pharmacogenetics of morphine poisoning in a breastfed neonate of a codeine-prescribed mother. Lancet. 2006;368:704. doi: 10.1016/S0140-6736(06)69255-6. [DOI] [PubMed] [Google Scholar]
- 11.Moretti ME, Koren G, Verjee Z, Ito S. Monitoring lithium in breast milk: an individualized approach for breast-feeding mothers. Ther Drug Monit. 2003;25:364–6. doi: 10.1097/00007691-200306000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Hale T, Kristensen J, Hackett L, Kohan R, Ilett K. Transfer of metformin into human milk. Adv Exp Med Biol. 2004;554:435–6. doi: 10.1007/978-1-4757-4242-8_58. [DOI] [PubMed] [Google Scholar]
- 13.Hale TW, Kristensen JH, Hackett LP, Kohan R, Ilett KF. Transfer of metformin into human milk. Diabetologia. 2002;45:1509–14. doi: 10.1007/s00125-002-0939-x. [DOI] [PubMed] [Google Scholar]
- 14.Hale TW, McDonald R, Boger J. Transfer of celecoxib into human milk. J Hum Lact. 2004;20:397–403. doi: 10.1177/0890334404269875. [DOI] [PubMed] [Google Scholar]
- 15.Ilett KF, Hale TW, Page-Sharp M, Kristensen JH, Kohan R, Hackett LP. Use of nicotine patches in breast-feeding mothers: transfer of nicotine and cotinine into human milk. Clin Pharmacol Ther. 2003;74:516–24. doi: 10.1016/j.clpt.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Kumar AR, Hale TW, Mock RE. Transfer of interferon alfa into human breast milk. J Hum Lact. 2000;16:226–8. doi: 10.1177/089033440001600308. [DOI] [PubMed] [Google Scholar]
- 17.Page-Sharp M, et al. Transfer of lamotrigine into breast milk. Ann Pharmacother. 2006;40:1470–1. doi: 10.1345/aph.1G667. [DOI] [PubMed] [Google Scholar]
- 18.Britton C, McCormick FM, Renfrew MJ, Wade A, King SE. Support for breastfeeding mothers. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD001141.pub3. CD001141. [DOI] [PubMed] [Google Scholar]
- 19.Miracle DJ, Fredland V. Provider encouragement of breastfeeding: efficacy and ethics. J Midwifery Womens Health. 2007;52:545–8. doi: 10.1016/j.jmwh.2007.08.013. [DOI] [PubMed] [Google Scholar]


