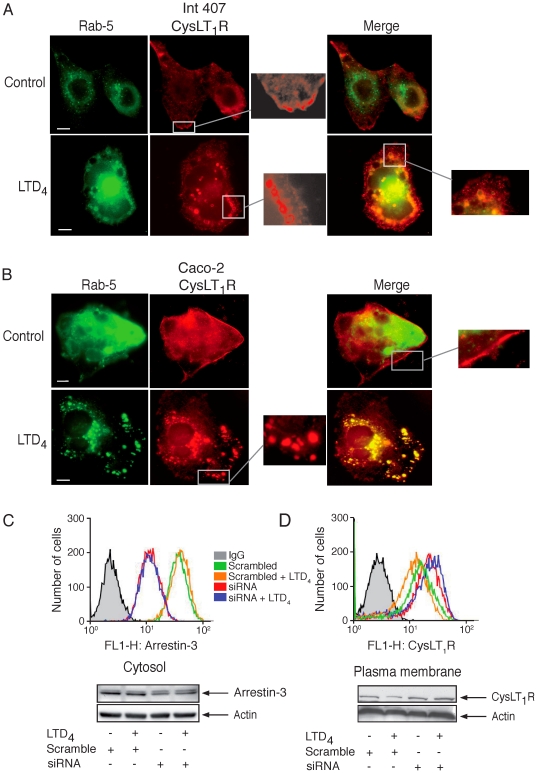
Figure 6. Co-localization of CysLT1R and Rab-5 protein in Int 407 and Caco-2 cells and arrestin-3-dependent internalization of the CysLT1R.
Fluorescent microscope images showing cells that were fixed, permeabilized, and stained with primary antibodies against Flag (1∶2500) using Alexa-546 conjugated secondary antibodies, Flag-CysLT1R, and GFP-Rab-5 in Int 407 cells (A) and Caco-2 cells (B). Cells were grown on cover slips to 50-60% confluency, transfected with Flag-CysLT1R and GFP-Rab-5, left to rest for 48 hours, and treated with or without 80 nM LTD4. The mounted slides were examined using a Nikon TE300 microscope (60× or 100×1.4 plan-apochromat oil immersion objective). (C, D) Cells were transfected, or not, with siRNA against arrestin-3 or scrambled siRNA, serum-starved, and stimulated, or not, with LTD4 (80 nM, 5 minutes). For FACS analysis, Int 407 cells (1×106 cells) were either first fixed and permabilized before intracellular staining for arrestin-3 or used directly for CysLT1R cell surface staining. Moreover, whole lysates or plasma membrane fractions were made and subjected to SDS-polyacrylamide gel electrophoresis and analyzed for arrestin-3 or CysLT1R protein expression using Western blot analysis. All membranes were re-probed for actin to ensure equal loading. The blots are representative of three separate experiments. The scale bar represents 10 µm.