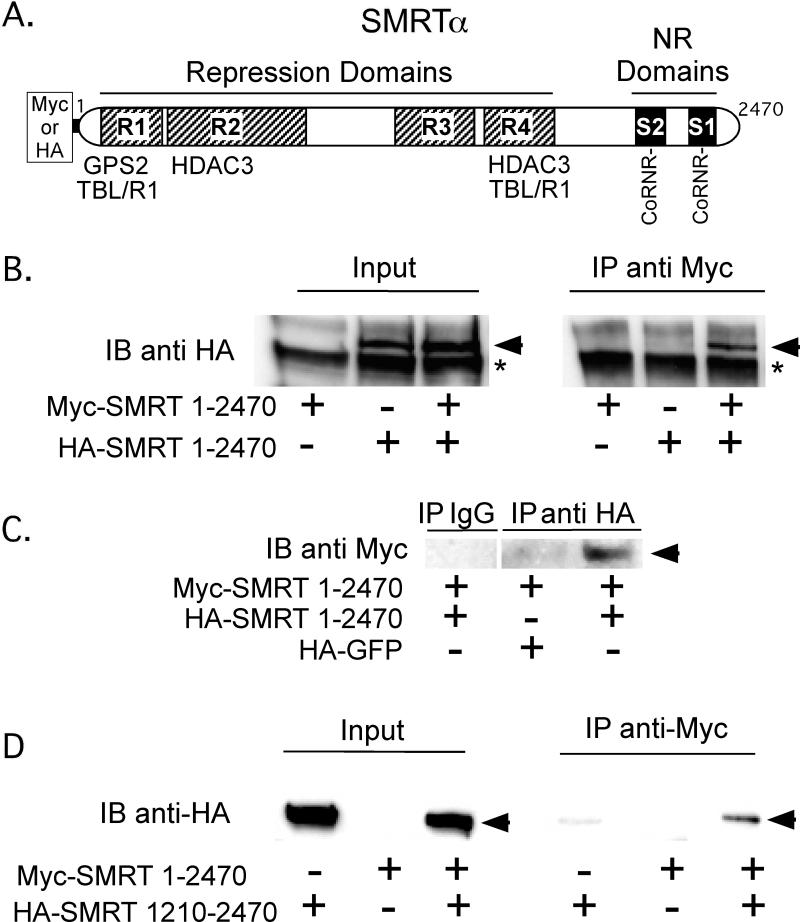
Figure 1. The SMRT corepressor, when expressed in cells, self-associates.
A. A schematic of the full-length SMRTα protein is shown. Repression domains (R1 to R4) and nuclear receptor (NR) interaction domains (S1 and S2) are indicated, as are the binding sites for GPS2, TBL1, TBLR1, and HDAC2, the CoRNR box nuclear receptor contact motifs, and the Myc or HA epitope tags employed for the co-immunoprecipitation experiments. B. SMRTα expressed in CV-1 cells self-associates. HA-tagged full-length SMRTα, Myc-tagged full-length SMRTα, or both were expressed by transient transfection of CV-1 cells. Cell lysates were then either analyzed directly by SDS-PAGE/immunoblot using anti-HA antibodies (left panel), or were first immunoprecipitated using anti-Myc antibodies and the immunoprecipitates were analyzed by SDS-PAGE/immunoblot using anti-HA antibodies (right panel). HA-tagged SMRTα is indicated by an arrowhead; * indicates a non-specific background protein. C. A reciprocal coimmunoprecipitation confirms SMRT self-association The experiment in panel B was repeated, but immunoprecipitating with anti-HA sera and immunoblotting with anti-Myc sera; HA-GFP was employed as a negative control. D. The C-terminal portion of the SMRTα protein is sufficient to mediate an interaction with full-length SMRTα. The protocol in panel B was repeated using a HA-tagged SMRTα (1210-2470) in place of full-length HA-SMRTα.