Abstract
Because of the increase in life expectancy, the contribution of age-related estrogen or androgen deficiency to obesity and type 2 diabetes will become a new therapeutic challenge. This review integrates current concepts on the mechanisms through which estrogen receptor (ER)s and androgen receptor (AR) regulate energy homeostasis in rodents and humans. In females, estrogen maintains energy homeostasis via ERα, and ERβ by suppressing energy intake and lipogenesis, enhancing energy expenditure and ameliorating insulin secretion and sensitivity. In males, testosterone is converted to estrogen and maintains fuel homeostasis via ERs and AR, which share related functions to suppress adipose tissue accumulation and improve insulin sensitivity. We argue that ERs and AR are targets to prevent age-related metabolic disorders.
Contribution of sex hormones to metabolic diseases
Increased food supply and decreased physical activity have resulted in a worldwide epidemic of obesity. As a consequence of these environmental changes, the incidence of type 2 diabetes (T2D) is on the rise [1]. In addition, a disorder involving increased visceral adipose tissue, hyperlipidemia, insulin resistance, and hypertension, namely, the metabolic syndrome, has emerged [2]. There is a concerted interaction between sex/reproduction and energy metabolism [3]. First, extreme conditions of disrupted energy balance such as obesity on one hand of the spectrum, or anorexia leading to cachexia on the other, both negatively impact fertility. Second, there are fundamental aspects of energy metabolism that are regulated differently in males and females [4]. To cite one critical example, female mammals bearing the burden of gestation and lactation have been favorably affected during evolution to resist the loss of body energy stores during prolonged periods of food scarcity and therefore deposit adipose tissue in the lower subcutaneous area, with lower lipolytic activity. Conversely, males deposit adipose tissue in visceral areas, with greater lipolytic activity to be able to mobilize energy stores promptly for muscle activity. It is believed that the circulating gonadal hormones, specifically androgen and estrogen, control these sex differences in energy balance between the onset of puberty and menopause. Because of the dramatic increase in life expectancy, women will spend the second half of their life, after menopause, in estrogen deficiency which predisposes to the metabolic syndrome and T2D [5]. Men will also spend a significant part of their life in age-related androgen deficiency.
Although no clear relationship exists between the gradual loss of T production and T2D, androgen deficiency clearly predisposes men to the metabolic syndrome [6]. Therefore, the contribution of sex hormone deficiency to metabolic diseases will become a new therapeutic challenge of the 21st century. Understanding how estrogen and androgen contribute to fuel homeostasis via their receptors promises to yield critical therapeutic applications. This review integrates current concepts on the role of estrogen, androgen and their receptors in regulating energy homeostasis in male and female rodents and humans. We also discuss how estrogen receptor(ER)s and androgen receptor (AR) are important targets for age-related metabolic disorders.
Estrogen receptors
Mechanism of ER action
In healthy premenopausal women, 17β-estradiol (E2) is produced by the ovaries by the aromatization of androstenedione to estrone, followed by conversion to E2. In these women, E2 functions as a circulating hormone that acts on distant target tissues. In post-menopausal women, however, when the ovaries fail to produce E2 and in men, E2 is produced in extra gonadal sites, mainly adipose tissue, bone, vessels and brain from the local tissue aromatization from circulating testosterone (T) [7]. Therefore, in males and females, T should be considered a circulating prohormone that is locally converted to either E2 acting on ERs, but also to 5α-dihydrotestosterone (DHT), the main ligand of the AR. Although DHT cannot be aromatized to estrogen, the situation is complicated by the fact that DHT can still be converted to a “second estrogen”, 5alpha-androstane-3beta,17beta-diol that acts on ERs [8]. The ER exists in two main forms, ERα and ERβ, which have multiple isoforms and exhibit distinct tissue expression patterns and functions [9]. In the classical ER signaling pathway, E2-activated ER binds as a homodimer to an estrogen response element (ERE) in target promoters or indirectly to an AP-1 or Sp-1 response element through association with other transcription factors, like Fos/Jun, that tether the activated ER to DNA [9]. This classical, “genomic” mechanism typically occurs within hours, leading to up- or down- regulation of gene transcription. E2 can also activate rapid signals, acting within minutes or seconds via extranuclear and membrane-associated forms of ERs and the G protein-coupled estrogen receptor (GPER), leading to activation of ion channels and protein kinases [10]. Although reproductive functions are mostly mediated via classical nuclear ERs acting as ligand-activated transcription factors, a large component of ER actions related to energy metabolism also involves extranuclear ERs, indirectly modulating gene expression or acting independently of nuclear events [11].
ERs control of energy intake and expenditure
The documented anti-obesity effects of E2 in vivo are centrally mediated. Surprisingly, the major models of estrogen deficiency and resistance do not exhibit hyperphagia. Thus, mice of both sexes lacking the aromatase enzyme, that cannot synthesize E2, develop obesity but show no hyperphagia or reduced energy expenditure. Rather, they exhibit a reduced spontaneous physical activity and a decrease in lean body mass [12]. Similarly, ERα deficiency in male and female mice causes obesity without hyperphagia but with decreased energy expenditure [13,14]. In male and female rats, Debbie Clegg made the observation that E2 enhances the ability of centrally administered leptin to suppress food intake [15]. This “leptino-mimetic” function of E2 is best observed in leptin-deficient (ob/ob) and leptin resistant (db/db) mice of both sexes, in which E2 decreases food intake and increases energy expenditure, resulting in a reduction in body weight [16]. The anorectic function of exogenously-administered E2 is present in female WT mice and is lost in female ERα-deficient mice, demonstrating that ERα activation is anorexigenic [17]. Thus, loss of ERα action produces a predominant decrease in energy expenditure while conversely, increasing ERα signaling by raising serum E2 concentrations both suppresses energy intake and increases energy expenditure. E2 also suppresses food intake through ERβ food intake since the anorectic effect of intracerebroventricular injection of E2 is blocked by co-administration of ERβ antisense oligodeoxynucleotides in female rats [18].
The precise anatomic site of ER suppression of body weight in the brain is still unknown. The arcuate nucleus (ARC) is a key hypothalamic area for mediating leptin’s inhibition of food intake. It contains first-order, leptin-responsive, anorexigenic pro-opiomelanocortin (POMC) neurons and orexigenic neuropeptide Y (NPY)/agouti-related peptide (AgRP) neurons. Tamas Horvath and coworkers showed that E2 triggers excitatory inputs to POMC neurons in the ARC of female rodents [16]. However, E2 does not seem to suppress food intake via action on ERs in ARC POMC neurons. In fact, it was recently suggested that E2 anorexigenic function in female mice is mediated via a decrease in hypothalamic orexigenic NPY and AgRP, but independent of ER action on ARC NPY and AgRP neurons, since these cells do not express ERα [19]. Most importantly, E2, acting via ERα in nucleus tractus solitarius neurons of the brainstem, is sufficient to inhibit feeding in female rats, suggesting that E2 anorectic function could originate in the hindbrain [20]. E2 stimulation of energy expenditure could involve both ERα and ERβ since silencing of ERα in the hypothalamus of female rodents reduces energy expenditure and produces obesity without hyperphagia [21], while the administration of an ERβ-selective agonist to high fat diet (HFD) fed female mice increases expression of the thermogenic uncoupling protein-1 in brown adipose tissue and reduces obesity [22]. Evidence points toward a site of action in the ventromedial nucleus of the hypothalamus (VMH) for ERα, since silencing of ERα in the VMH reduces energy expenditure [21]. Unlike in the case of ERα and ERβ, the role of G protein-coupled ER (GPER) in body weight regulation still needs validation. In one study, female mice lacking GPER developed obesity [23]. However, the obesity phenotype was observed in only one of the four GPER mutant mouse lines studied [24]. The signaling mechanisms of ER actions in hypothalamic neurons are not fully elucidated, but available evidence suggests that it involves extranuclear ERs. First, E2 triggers a rapid increase in excitatory inputs to POMC neurons in the ARC in vivo, consistent with rapid, extranuclear or membrane initiated actions [16], and accordingly, E2 can suppress NPY in clonal hypothalamic neurons via a membrane form of ERα [25]. Second, an E2-responsive, Gq-coupled membrane receptor (Gq-mER) is involved in mediating the anorectic effects of E2 on food intake and body temperature in hypoestrogenic female rodents [26,27].
ERs suppress lipogenesis in white adipose tissue and liver
E2 also suppresses white adipose tissue (WAT) accumulation by decreasing fatty acid and triglyceride synthesis or lipogenesis. Greenberg and coworkers showed that in vivo treatment with E2 reduces adipocyte size in ovariectomized female mice by reducing fatty acid uptake (down-regulation of lipoprotein lipase), reducing lipogenesis (down-regulation of acetyl-coA carboxylate and fatty acid synthase), and increasing catecholamine-stimulated lipolysis [28]. Similarly, E2 suppresses lipogenic genes and triglyceride accumulation in WAT and liver in HFD-fed [29] and leptin-resistant female mice [30]. Interestingly, this effect is reproduced by ERβ, but not ERα, selective agonists [22,31]. ERs are expressed in adipocytes and hepatocytes of both sexes, and extensive evidence demonstrates that E2 has direct effects on cultured adipocytes with the overall effect of inhibiting adipogenesis and lipogenesis [32]. Thus, the E2 effects described above could result from ER action in peripheral tissues. Still, the exact contribution of E2 anti-lipogenic effects in vivo resulting from direct ER action in WAT and liver or from central ER action affecting adipose and liver via the autonomous system is still unknown. Although the overall effect of E2 is to decrease WAT accumulation, E2 favors subcutaneous WAT accumulation via central [15] and peripheral mechanisms in both sexes [32]. ERβ is anti-lipogenic and anti-adipogenic. ERβ-deficiency favors WAT accumulation in female mice during high fat feeding by increasing PPARγ signaling in WAT, thus demonstrating that ERβ acts directly on adipocytes in vivo and is a negative regulator of PPARγ [33]. In addition, ERβ–selective ligands show PPARγ antagonistic actions in adipocytes mediated though a mechanism involving ERβ competing with PPARγ for peroxisome-proliferator-activated receptor-γ coactivator 1 (PGC1)α [22].
ERs improve insulin sensitivity
E2, at physiological concentrations, favors insulin sensitivity, and E2 deficiency and/or resistance provokes insulin resistance. Perhaps the best evidence is that men lacking E2 production secondary to mutations in the aromatase gene or men harboring E2 resistance secondary to genetic ERα deficiency develop insulin resistance and/or glucose intolerance [34,35]. Accordingly, male and female mice with E2 deficiency or E2 resistance by elimination of the aromatase or ERα genes develop insulin resistance [12,13]. The cause of insulin resistance induced by E2 deficiency or resistance is probably multifactorial. In one study, female mice lacking ERα did not show insulin resistance in skeletal muscle but exhibited decreased insulin suppression of hepatic glucose production (HGP) during a euglycemic, hyperinsulinemic clamp in anesthetized mice, suggesting that ERα deficiency provokes hepatic insulin resistance [36]. Andrea Hevener and coworkers, however, reported that ERα-deficient female mice accumulate pro-inflammatory lipid intermediates in skeletal muscle leading to marked muscle insulin resistance with minor alterations in liver insulin sensitivity during euglycemic, hyperinsulinemic clamp conditions in conscious mice [14]. In addition, decreased expression of the insulin-sensitive glucose transporter GLUT4 is observed in skeletal muscle of male ERα-deficient mice, which may contribute to the muscle insulin resistance observed in these mice since GLUT4 is essential to insulin-sensitive glucose transport in skeletal muscle and WAT [37]. E2 treatment improves insulin resistance in female mice fed a high fat diet [29,38] and in obese female mice with genetic leptin resistance [30] through a pathway at least partially dependent on ERα [31,38]. E2 treatment also reduces HFD-induced insulin resistance in skeletal muscle by fifty percent during hyperinsulinemic euglycemic clamp in an ERα-dependent manner [38]. However, as discussed, E2 also suppresses lipogenesis and steatosis in liver of HFD-fed [29] and leptin resistant mice [30] suggesting that it protects from insulin resistance by preventing ectopic lipid accumulation (lipotoxicity). In summary, ERα deficiency decreases GLUT4 expression in skeletal muscle and impairs lipid homeostasis in skeletal muscle and liver of rodents, thus decreasing insulin’s ability to suppress HGP and to promote skeletal muscle glucose utilization. Accordingly, activation of ERα during HFD and genetic leptin resistance improves insulin resistance induced by ectopic lipid accumulation in skeletal muscle [29,30,31,38]. Still, the effect of ERα in mediating insulin sensitivity via central mechanisms remains to be determined. In absence of ERα signaling, ERβ could promote insulin resistance in skeletal muscle. Ovariectomy in hyperestrogenic female ERα-deficient mice (which suppresses E2 action though ERβ), improves glucose tolerance and insulin sensitivity [39] and administration of an ERβ-selective agonist in male E2 deficient ArKO mice decreases skeletal muscle GLUT4 expression [37]. Accordingly, administration of tamoxifen, acting as an ERβ antagonist in male ERα-deficient mice, increased GLUT4 expression and improved insulin sensitivity [40]. Interestingly, ERs modulate GLUT4 expression in WAT and skeletal muscle in a tissue-specific way. While ERβ-mediated repression of GLUT4 predominates in skeletal muscle, ERα-mediated induction of GLUT4 predominates in WAT [40].
Finally, recent evidence indicates that ERβ-deficiency protects against diet-induced insulin resistance in male mice by increasing PPARγ signaling in adipose tissue, which indirectly improves skeletal muscle insulin action by promoting lipid accumulation away from muscle and into adipose tissue [33].
The physiological and genetic evidence argues that E2 and ERα favor insulin sensitivity in rodents and humans of both sexes when E2 concentrations stay within a tight physiological window. Conversely, high doses of estrogens provoke insulin resistance [41,42]. In fact, two recent studies have reported that in postmenopausal women, higher plasma levels of E2 (associated with higher T levels) were strongly and prospectively related to increased risk of developing T2D [43,44].
ERs favor β-cell function and survival
The beneficial effect of estrogens on β-cell function in humans and rodents has been recently reviewed [11]. We will focus on the most important and recent developments. There are gender dimorphisms in rodent models of β-cell failure that helped us identify the function of ERs in β-cells. A classical sexually-dimorphic model of T2D is the transgenic mouse overexpressing human amyloid polypeptyde (hIAPP) in pancreatic β-cells. The hIAPP is a classical late β-cell injury in T2D. Steven Kahn and co-workers initially reported that overexpression of hIAPP in islets predisposes mice to the development of islet amyloid and hyperglycemia with a strong male predominance [45]. This led to the paradigm that suppression of E2 production by ovariectomy enhanced islet amyloid formation in female mice [46] and that conversely, E2 treatment prevents amyloid formation and β-cell failure in males [47]. In order to study the anti-apoptotic action of E2 on islets in vivo, we used the mouse model of β-cell injury induced by streptozotocin (STZ) and showed that circulating E2 acts as a protective hormone, preventing β-cell apoptosis in vivo in both sexes and at physiological concentrations [48]. ERα and ERβ are expressed in rodent and human β-cells in both sexes where they exhibit a predominant extranuclear localization [48,49,50]. E2-activated ERα prevents islet apoptosis in males and females via an ERE-independent pathway [50]. This is mediated via activation of extra-nuclear ERs with a predominant ERα effect [50]. Although the precise signaling pathways are still under investigation, it appears that ERα and ERβ prevent apoptosis via distinct pathways, independently of gene transcription or de novo protein synthesis suggesting that this cytoprotection happens independently of nuclear events [50,51].
GPER is present in β-cells and in one study, GPER-deficient mice displayed altered insulin release from isolated islets stimulated with pharmacological concentrations of E2. In this study, impaired glucose-stimulated insulin secretion (GSIS) was observed in GPER-deficient mice but the mice were chronically treated with E2 and insulin sensitivity was not assessed [52]. In another GPER-deficient mouse, however, we did not observe any alteration in GSIS [50]. Thus, the role of GPER in GSIS remains controversial.
Elimination of GPER predisposes to STZ-induced islet apoptosis in female mice, but not in males [50]. We and others observed that pharmacological activation of GPER by the agonist, G1, prevents oxidative stress and cytokine-induced apoptosis in cultured mouse and human islets [50,53]. G1 has recently been shown to induce the expression and to activate a small 36kDa ERα isoform lacking transcriptional activity and mediating rapid estrogen signaling, suggesting that GPER signals as an inducer of ERα36 [54]. However, the observation that G1 cytoprotection is lost in cultured GPER deficient islets further supports the functional significance of GPER itself in islet survival [50]. Recently, Nadal and co-workers reported that E2 activation of ERβ enhances GSIS in cultured islets by suppressing the ATP-sensitive potassium channel through effects on the membrane atrial natriuretic peptide receptor [55]. This finding shed new light on the role of ERβ in islet function.
The Zucker Diabetic Fatty (ZDF) rat is a classical model of T2D and a critical example of sex dimorphism. Male ZDF rats develop pancreatic β-cell failure to compensate for insulin resistance leading to overt T2D [56]. Fifteen years ago Roger Unger reported that β-cell failure in male ZDF rats is secondary to islet triglyceride accumulation leading to β-cell apoptosis, and the concept of lipotoxicity was born [57]. β-cell failure occurs almost exclusively in male ZDF rats, while female ZDF rats remain normoglycemic [57]. Interestingly, islet triglyceride content in the adult ZDF female is 70% lower than that of males [57] suggesting that E2 prevents islet lipid accumulation. Indeed, we recently reported that E2 treatment of male ZDF rats suppresses islet lipogenesis and prevents β-cell failure probably via ERα action in islets [58].
ERα is also important for insulin biosynthesis. We and others have shown that exposure to physiological concentrations of E2 increases β-cell insulin gene expression and insulin content via an extranuclear ERα-dependent mechanism involving Src and ERK kinases [49,59] and an increase in NeuroD-1 binding to the insulin promoter [59]. Thus, the elevated E2 concentration during pregnancy may participate in the islet adaptation to the increased metabolic demand by enhancing insulin biosynthesis and release via ERα and ERβ [49,55,59]. In conclusion, E2 at physiological concentrations increases insulin production and protects the pancreatic β-cells against major β-cell injuries encountered in diabetes, such as lipotoxicity, hIAPP, oxidative stress, and apoptosis.
Most ERα actions that control body weight, insulin sensitivity, and β-cell biology [12,13,14,15,32,34,35,37,40,48,50] are present in both sexes, demonstrating that T aromatization in E2 acting on ERα is important to energy homeostasis in males. However, the role of ERβ has been studied in both sexes for β-cell survival only [50], and for either females for body weight regulation [18,22,33] or males for insulin sensitivity [37,40 ]. Therefore it is assumed that ERα and ERβ share similar metabolic function in both sexes. Figures 1 and 2 summarize these actions in women and men, respectively.
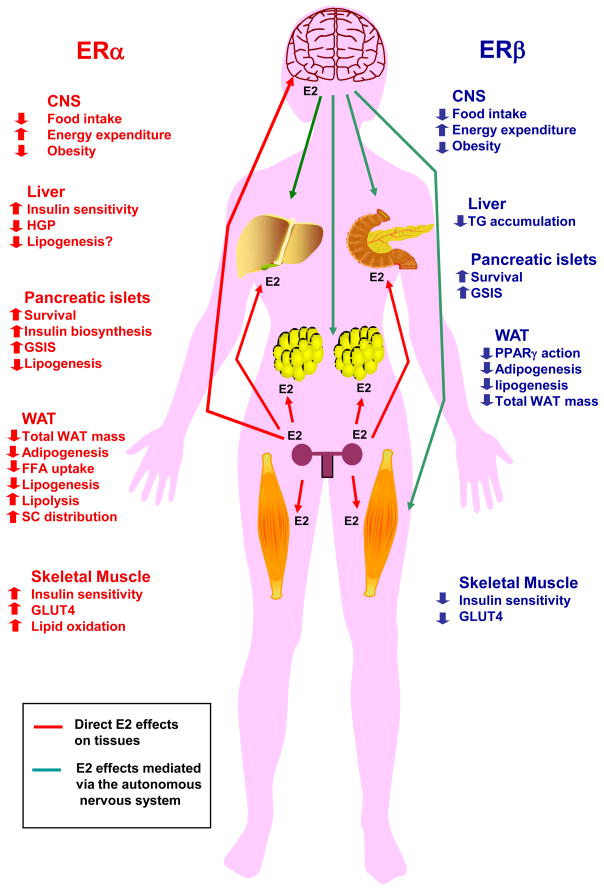
Figure 1. Metabolic effects of ERα and ERβ activation in females.
Activation of ERα in the central nervous system (CNS) suppresses food intake, increases energy expenditure and decreases body weight. In addition, activation of ERα improves peripheral energy and glucose homeostasis in multiple ways by 1) preventing liver steatosis, suppressing hepatic glucose production and improving insulin sensitivity, 2) enhancing skeletal muscle lipid oxidation, GLUT4 expression and insulin sensitivity, 3) enhancing subcutaneous white adipose tissue (WAT) distribution while decreasing overall WAT mass by decreasing WAT free fatty acid (FFA) uptake, lipid synthesis and increasing lipolysis, 4) favoring pancreatic β-cell survival and function by preventing pro-apoptotic injuries and lipotoxicity, and increasing insulin biosynthesis and glucose-stimulated insulin release (GSIS). Activation of ERβ in the central nervous system (CNS) also suppresses food intake and increases energy expenditure and prevents obesity on a high fat diet. In addition, activation of ERβ affects peripheral energy and glucose homeostasis by 1) favoring pancreatic β-cell survival and function by preventing pro-apoptotic injuries and increasing GSIS, 2) preventing obesity and decreasing WAT mass, 3) promoting insulin resistance in absence of ERα activation. ERα and ERβ metabolic actions on peripheral tissues result from direct activations of ERs in these tissues or from a central ER action affecting peripheral tissues via the autonomous system CNS ERs.
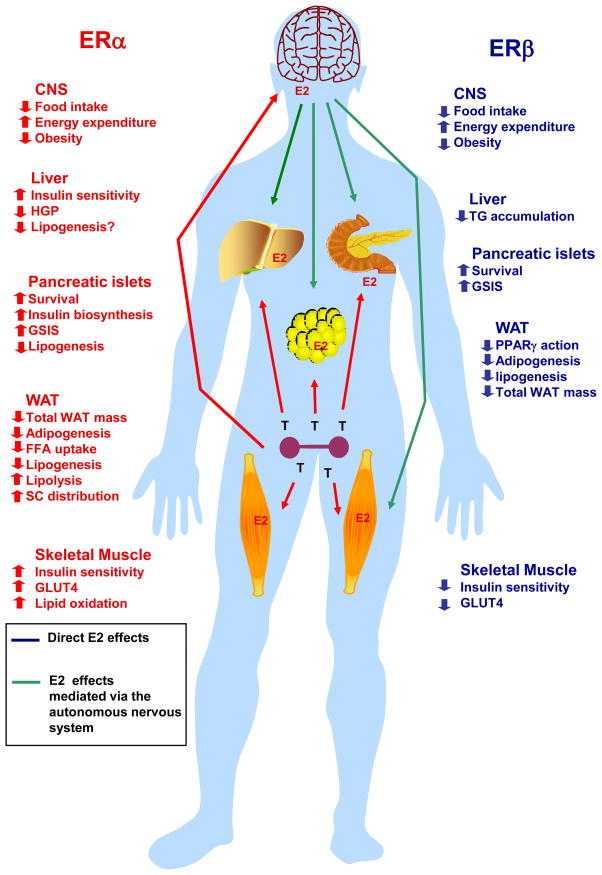
Figure 2. Metabolic effects of ERα and ERβ activation in males.
Activation of ERα in males has similar effect than in females. In the central nervous system (CNS), ERα suppresses food intake, increases energy expenditure and decreases body weight. In addition, activation of ERα improves peripheral energy and glucose homeostasis in multiple ways by 1) preventing liver steatosis, suppressing hepatic glucose production and improving insulin sensitivity, 2) enhancing skeletal muscle lipid oxidation, GLUT4 expression and insulin sensitivity, 3) enhancing subcutaneous white adipose tissue (WAT) distribution while decreasing overall WAT mass by decreasing WAT free fatty acid (FFA) uptake, lipid synthesis and increasing lipolysis, 4) favoring pancreatic β-cell survival and function by preventing pro-apoptotic injuries and lipotoxicity, and increasing insulin biosynthesis and glucose-stimulated insulin release (GSIS). Activation of ERβ in the central nervous system (CNS) also suppresses food intake and increases energy expenditure and prevents obesity on a high fat diet. In addition, activation of ERβ affects peripheral energy and glucose homeostasis by 1) favoring pancreatic β-cell survival and function by preventing pro-apoptotic injuries and increasing GSIS, 2) preventing obesity and decreasing WAT mass, 3) promoting insulin resistance in absence of ERα activation. ERα and ERβ metabolic actions on peripheral tissues result from direct activations of ERs in these tissues or from a central ER action affecting peripheral tissues via the autonomous system CNS ERs.
Androgen Receptor
Mechanism of AR action
Androgens influence gene transcription through the activation of the androgen receptor (AR), a ligand-activated transcription factor that subsequently binds as a homodimer with specific DNA motifs in its target genes [60]. These DNA motifs, called androgen response elements (AREs), can be classified as classical AREs, which are recognized by glucocorticoid or progesterone receptors and AR-specific AREs, which display selectivity for the AR [61]. As in the case of estrogens, over the past two decades evidence has accumulated to implicate rapid responses to androgens, dependent or independent of the AR [62].
AR prevents visceral fat accumulation in males
T deprivation in men contributes to the development of the metabolic syndrome. There is an inverse relationship between total serum testosterone and the amount of visceral adipose tissue and the metabolic syndrome [63]. This is observed in the context of age-related hypogonadism [64], inherited T deficiency [65], and androgen deprivation during treatment of prostate cancer [66].
Accordingly, in men, high T is linked to insulin sensitivity [67]. Evidence discussed in the ER section demonstrates that aromatization of T into E2 is critical to energy homeostasis in males, suggesting that T acts as a prohormone in men to provide E2 for tissue energy homeostasis. Indeed, orchidectomized male rodents treated with either T or E2 remain lean, while those treated with the pure androgen DHT (that is not aromatized to E2), develop obesity [68]. Several lines of evidence demonstrate, however, that T has anti-obesity actions that are mediated via AR. First, men with genetic androgen resistance linked to CAG repeats in the AR gene, which decreases AR-mediated gene transcription, have elevated visceral fat [69]. Second, male mice lacking AR develop late onset visceral obesity with increased lipogenesis in WAT and liver [70,71]. Furthermore, AR is involved in adiponectin biology. Adiponectin is high in hypogonadal men and reduced by T therapy [72]. T infusion also decreases adiponectin in mice [73], an effect that is at least partially mediated via AR since adiponectin is increased in AR-deficient mice [70]. Whether AR suppression of adiponectin reflects increased adiponectin sensitivity or a decreased adipocyte number remains to be determined.
The suppressing effect of T on WAT mass in males may be indirectly mediated via AR signaling in skeletal muscle. Several lines of evidence support this scenario. First, in vitro, T promotes the commitment of pluripotent mesenchymal stem cells into myogenic lineage while inhibiting the adipogenic lineage via an AR-dependent mechanism [74] mediated via non-canonical Wnt signaling [75]. This androgenic anabolism involves an induction of IGF1, leading to nuclear accumulation of beta-catenin, a pro-myogenic, anti-adipogenic stem cell regulatory factor [76]. Accordingly, selective overexpression of AR in muscle cells of transgenic male rats increases lean mass with hypertrophy of type IIb fibers, increasing oxidative metabolism thus decreasing adipocyte size and WAT mass [77]. Conversely, and consistent with this model, male mice lacking AR in adipose tissue are not obese. These mice show an increased WAT production of leptin without leptin resistance [78]. Thus, activation of AR in skeletal muscle may indirectly decrease WAT mass by increasing muscle oxidative metabolism or through the release of a circulating factor.
AR action in skeletal muscle promotes insulin sensitivity in males
Apart from increasing visceral WAT, the mechanism of AR deficiency-induced insulin resistance probably involves a decrease in the transcription factor PGC1α in skeletal muscle. Indeed, PGC1α stimulates mitochondrial biogenesis and skeletal muscle oxidative fibers and is thus a molecular marker of muscle insulin sensitivity. A decrease in PGC1α expression in skeletal muscle of T2D subjects is associated with insulin resistance [79]. Similarly, in men, low T levels are associated with low PGC1α expression levels in muscle [67] and AR-deficient mice have low levels of PGC1α in tissues [70]. Thus, T deficiency promotes insulin resistance at least partially via an AR-dependent mechanism involving a decrease in PGC1α-mediated oxidative and insulin sensitive muscle fibers as well as increased visceral WAT and liver steatosis.
Central AR actions favor energy homeostasis in males
AR is expressed in the brain more abundantly in males [80]. Male AR-deficient mice develop obesity without hyperphagia but with reduced locomotor activity and energy expenditure associated with decreased brown adipose tissue thermogenesis [70]. AR suppresses lipogenesis in males, and male AR-deficient mice exhibit unsuppressed lipogenesis in muscle and liver [70,71]. AR also functions in the male hypothalamus to favor central leptin signaling. AR-deficient male mice exhibit a failure of leptin to promote STAT3 nuclear localization in ARC neurons and to suppress food intake and reduce body weight even before the onset of overt obesity [80]. In summary, in males, AR is involved in the control of WAT mass via central and peripheral effects.
AR and β-cells in males
Early studies reported that T accelerates the hyperglycemic decompensation via an AR-dependent mechanism in male mouse models of insulin-deficient diabetes in which β-cell destruction is induced by streptozotocin [81,82]. Recently, however, it was reported that testosterone protects early apoptotic damage induced by streptozotocin in male rat pancreas and via an AR dependent mechanism [83,84]. In the later study, however, the effect of T on diabetes incidence was not reported. Therefore the role of the AR in male β-cell survival and function needs clarification. Figure 3 summarizes AR’s effects on energy homeostasis in men.
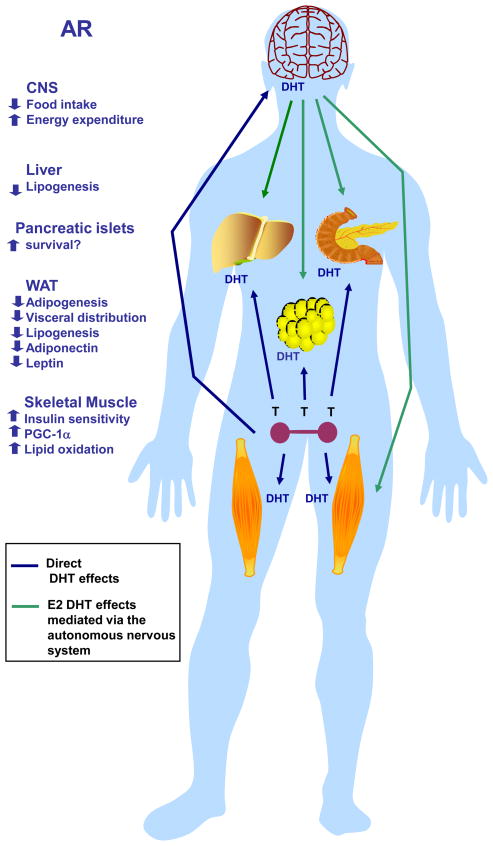
Figure 3. Metabolic effects of AR activation in males.
Physiological activation of AR in the central nervous system (CNS) suppresses food intake, increases energy expenditure and decreases body weight. In addition, physiological activation of AR improves peripheral energy and glucose homeostasis by 1) preventing liver steatosis 2) enhancing skeletal muscle insulin sensitivity by increasing PGC1α expression, mitochondrial biogenesis and skeletal muscle oxidative fibers, thus increasing lipid oxidation, 3) decreasing WAT lipogenesis and visceral WAT mass. AR activation also decreases WAT adiponectin and leptin production.
Role of AR in females
The role of AR in female energy metabolism is not well characterized. While AR deficiency is reported to have no effect on body weight in female mice [85], women with complete androgen insensitivity syndrome have increased total fat mass compared to both female and male age-matched control subjects [86]. Therefore further studies are needed to determine the role of AR in female energy metabolism.
Although the consequence of AR deficiency in females in not well studied, the association between hyperandrogenicity and diabetes in women has been known for almost a century [87]. It has been postulated that excess androgen provokes insulin resistance. In women, hyperandrogenism is a risk factor for the metabolic syndrome independently of obesity and insulin resistance [88]. Furthermore, T infusion in healthy women decreases insulin-stimulated whole body glucose uptake [89]. The role of excess T in promoting skeletal muscle insulin resistance with fiber type switch has also been confirmed from studies in female rodents [90]. Hyperandrogenemia is also associated with pancreatic β-cell dysfunction [91,92,93]. In some studies of women with PCOS, β-cell dysfunction is closely associated with the degree of androgenicity, independent of insulin resistance, raising the possibility that excess T may predispose to secondary β-cell failure [92,93]. Consistent with this hypothesis, in mice, T accelerates the hyperglycemic decompensation in experimental models of insulin-dependent diabetes in which β-cell destruction is induced by oxidative stress or inflammation [81,94]. In addition, hyperandrogenemia in women with PCOS is accompanied by systemic oxidative stress [95], and excess T in female mice similarly provokes systemic oxidative stress via an AR-dependent mechanism [94]. We showed that in the presence of a prior β-cell injury, excess T predisposes female mice to β-cell failure via an AR-dependent mechanism [94] that could involve an AR present in β-cells [96]. Thus, excess AR activation in β-cells may participate in β-cell dysfunction observed in women with androgen excess.
Despite accumulated evidence that T excess alters metabolism in females, it is not clear whether T excess initiates metabolic abnormalities or perpetuates them. Indeed, treatment with AR antagonists or suppression of ovarian androgen production with GnRH analogues in hyperandrogenic women does not always improve insulin resistance [97], thereby suggesting that excess androgen in women may not be instrumental in the metabolic abnormalities but rather an aggravating factor. Further studies in this area are needed.
Conclusions and perspectives
E2 and T are critical hormonal signals maintaining energy homeostasis in both sexes, and the impact of E2 treatment on obesity and diabetes prevention is one of the most powerful observations of rodent physiology. Although men have lower circulating E2 concentrations than premenopausal women, aromatization of circulating T to E2 in target metabolic tissues equilibrates cellular E2 concentrations, and ER activation is similarly critical in both sexes in promoting fuel homeostasis. Conversely, and probably reflecting the lower circulating and cellular T and DHT concentrations in females, AR activation is weak in females and thus AR is less important. Indeed, if androgen concentrations increase in females to the level of males, this provokes excess AR activation leading to metabolic disturbances. The mechanism of this bi-directional modulation of metabolism by AR between males and females is unknown. Because of this sex-specific stoichoimetry of ERs/AR activation, AR is primarily a male drug target, while ERs are sex non-specific drug targets to improve metabolic diseases. The major obstacle to the development of ER and AR ligands to treat metabolic diseases is the fear of hormone-dependent cancer. Further studies are thus needed to identify and develop new ligands that prevent diabetes and obesity that lack the mitogenic actions predisposing to hormone-dependent cancers. This can be achieved by targeting E2 or T to the appropriate cells or developing novel selective ER/AR modulators that retain the beneficial effects of their ligand in selected tissues while lacking the mitogenic actions in reproductive organs.
Acknowledgments
This work was supported by grants from National Institutes of Health (P50 HD044405, RO1 DK074970-01), the Juvenile Diabetes Research Foundation (1-2006-837), and the March of Dimes (6-FY07-678) and by Northwestern University Institute for Women’s Health Research Pioneer Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 3.Hill JW, Elmquist JK, Elias CF. Hypothalamic pathways linking energy balance and reproduction. Am J Physiol Endocrinol Metab. 2008;294:E827–832. doi: 10.1152/ajpendo.00670.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi H, Seeley RJ, Clegg DJ. Sexual differences in the control of energy homeostasis. Front Neuroendocrinol. 2009;30:396–404. doi: 10.1016/j.yfrne.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88:2404–2411. doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- 6.Zitzmann M. Testosterone deficiency, insulin resistance and the metabolic syndrome. Nat Rev Endocrinol. 2009;5:673–681. doi: 10.1038/nrendo.2009.212. [DOI] [PubMed] [Google Scholar]
- 7.Simpson ER. Sources of estrogen and their importance. J Steroid Biochem Mol Biol. 2003;86:225–230. doi: 10.1016/s0960-0760(03)00360-1. [DOI] [PubMed] [Google Scholar]
- 8.Weihua Z, Lathe R, Warner M, Gustafsson JA. An endocrine pathway in the prostate, ERbeta, AR, 5alpha-androstane-3beta,17beta-diol, and CYP7B1, regulates prostate growth. Proc Natl Acad Sci U S A. 2002;99:13589–13594. doi: 10.1073/pnas.162477299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, et al. Mechanisms of estrogen action. Physiol Rev. 2001;81:1535–1565. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- 10.Levin ER. Plasma membrane estrogen receptors. Trends Endocrinol Metab. 2009;20:477–482. doi: 10.1016/j.tem.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu S, Mauvais-Jarvis F. Minireview: Estrogenic protection of beta-cell failure in metabolic diseases. Endocrinology. 2010;151:859–864. doi: 10.1210/en.2009-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones ME, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, et al. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci U S A. 2000;97:12735–12740. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ribas V, Nguyen MT, Henstridge DC, Nguyen AK, Beaven SW, et al. Impaired Oxidative Metabolism and Inflammation are Associated with Insulin Resistance in ER{alpha} Deficient Mice. Am J Physiol Endocrinol Metab. 2009;298:E304–19. doi: 10.1152/ajpendo.00504.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978–987. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- 16.Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, et al. Anorectic estrogen mimics leptin’s effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med. 2007;13:89–94. doi: 10.1038/nm1525. [DOI] [PubMed] [Google Scholar]
- 17.Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa S. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-alpha null mice. Endocrinology. 2001;142:4751–4757. doi: 10.1210/endo.142.11.8504. [DOI] [PubMed] [Google Scholar]
- 18.Liang YQ, Akishita M, Kim S, Ako J, Hashimoto M, et al. Estrogen receptor beta is involved in the anorectic action of estrogen. Int J Obes Relat Metab Disord. 2002;26:1103–1109. doi: 10.1038/sj.ijo.0802054. [DOI] [PubMed] [Google Scholar]
- 19.Olofsson LE, Pierce AA, Xu AW. Functional requirement of AgRP and NPY neurons in ovarian cycle-dependent regulation of food intake. Proc Natl Acad Sci U S A. 2009;106:15932–15937. doi: 10.1073/pnas.0904747106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thammacharoen S, Lutz TA, Geary N, Asarian L. Hindbrain administration of estradiol inhibits feeding and activates estrogen receptor-alpha-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology. 2008;149:1609–1617. doi: 10.1210/en.2007-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, et al. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci U S A. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yepuru M, Eswaraka J, Kearbey JD, Barrett CM, Raghow S, et al. Estrogen receptor-{beta} selective ligands alleviate high-fat diet- and ovariectomy-induced obesity in mice. J Biol Chem. 2010;285:31292–303. doi: 10.1074/jbc.M110.147850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haas E, Bhattacharya I, Brailoiu E, Damjanovic M, Brailoiu GC, et al. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ Res. 2009;104:288–291. doi: 10.1161/CIRCRESAHA.108.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langer G, Bader B, Meoli L, Isensee J, Delbeck M, et al. A critical review of fundamental controversies in the field of GPR30 research. Steroids. 2010;75:603–610. doi: 10.1016/j.steroids.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Dhillon SS, Belsham DD. Estrogen inhibits NPY secretion through membrane-associated estrogen receptor (ER)-alpha in clonal, immortalized hypothalamic neurons. Int J Obes (Lond) 2010 Jun 15; doi: 10.1038/ijo.2010.124. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Qiu J, Bosch MA, Tobias SC, Krust A, Graham SM, et al. A G-protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci. 2006;26:5649–5655. doi: 10.1523/JNEUROSCI.0327-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roepke TA, Bosch MA, Rick EA, Lee B, Wagner EJ, et al. Contribution of a Membrane Estrogen Receptor to the Estrogenic Regulation of Body Temperature and Energy Homeostasis. Endocrinology. 2010;15:4926–37. doi: 10.1210/en.2010-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D’Eon TM, Souza SC, Aronovitz M, Obin MS, Fried SK, et al. Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J Biol Chem. 2005;280:35983–35991. doi: 10.1074/jbc.M507339200. [DOI] [PubMed] [Google Scholar]
- 29.Bryzgalova G, Lundholm L, Portwood N, Gustafsson JA, Khan A, et al. Mechanisms of antidiabetogenic and body weight-lowering effects of estrogen in high-fat diet-fed mice. Am J Physiol Endocrinol Metab. 2008;295:E904–912. doi: 10.1152/ajpendo.90248.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao H, Bryzgalova G, Hedman E, Khan A, Efendic S, et al. Long-term administration of estradiol decreases expression of hepatic lipogenic genes and improves insulin sensitivity in ob/ob mice: a possible mechanism is through direct regulation of signal transducer and activator of transcription 3. Mol Endocrinol. 2006;20:1287–1299. doi: 10.1210/me.2006-0012. [DOI] [PubMed] [Google Scholar]
- 31.Lundholm L, Bryzgalova G, Gao H, Portwood N, Falt S, et al. The estrogen receptor {alpha}-selective agonist propyl pyrazole triol improves glucose tolerance in ob/ob mice; potential molecular mechanisms. J Endocrinol. 2008;199:275–286. doi: 10.1530/JOE-08-0192e. [DOI] [PubMed] [Google Scholar]
- 32.Cooke PS, Naaz A. Role of estrogens in adipocyte development and function. Exp Biol Med (Maywood) 2004;229:1127–1135. doi: 10.1177/153537020422901107. [DOI] [PubMed] [Google Scholar]
- 33.Foryst-Ludwig A, Clemenz M, Hohmann S, Hartge M, Sprang C, et al. Metabolic actions of estrogen receptor beta (ERbeta) are mediated by a negative cross-talk with PPARgamma. PLoS Genet. 2008;4:e1000108. doi: 10.1371/journal.pgen.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab. 1995;80:3689–3698. doi: 10.1210/jcem.80.12.8530621. [DOI] [PubMed] [Google Scholar]
- 35.Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331:1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 36.Bryzgalova G, Gao H, Ahren B, Zierath JR, Galuska D, et al. Evidence that oestrogen receptor-alpha plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia. 2006;49:588–597. doi: 10.1007/s00125-005-0105-3. [DOI] [PubMed] [Google Scholar]
- 37.Barros RP, Machado UF, Warner M, Gustafsson JA. Muscle GLUT4 regulation by estrogen receptors ERbeta and ERalpha. Proc Natl Acad Sci U S A. 2006;103:1605–1608. doi: 10.1073/pnas.0510391103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riant E, Waget A, Cogo H, Arnal JF, Burcelin R, et al. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology. 2009;150:2109–2117. doi: 10.1210/en.2008-0971. [DOI] [PubMed] [Google Scholar]
- 39.Naaz A, Zakroczymski M, Heine P, Taylor J, Saunders P, et al. Effect of ovariectomy on adipose tissue of mice in the absence of estrogen receptor alpha (ERalpha): a potential role for estrogen receptor beta (ERbeta) Horm Metab Res. 2002;34:758–763. doi: 10.1055/s-2002-38259. [DOI] [PubMed] [Google Scholar]
- 40.Barros RP, Gabbi C, Morani A, Warner M, Gustafsson JA. Participation of ERalpha and ERbeta in glucose homeostasis in skeletal muscle and white adipose tissue. Am J Physiol Endocrinol Metab. 2009;297:E124–133. doi: 10.1152/ajpendo.00189.2009. [DOI] [PubMed] [Google Scholar]
- 41.Barros RP, Machado UF, Gustafsson JA. Estrogen receptors: new players in diabetes mellitus. Trends Mol Med. 2006;12:425–431. doi: 10.1016/j.molmed.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Nadal A, Alonso-Magdalena P, Soriano S, Quesada I, Ropero AB. The pancreatic beta-cell as a target of estrogens and xenoestrogens: Implications for blood glucose homeostasis and diabetes. Mol Cell Endocrinol. 2009;304:63–68. doi: 10.1016/j.mce.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 43.Ding EL, Song Y, Manson JE, Rifai N, Buring JE, et al. Plasma sex steroid hormones and risk of developing type 2 diabetes in women: a prospective study. Diabetologia. 2007;50:2076–2084. doi: 10.1007/s00125-007-0785-y. [DOI] [PubMed] [Google Scholar]
- 44.Kalyani RR, Franco M, Dobs AS, Ouyang P, Vaidya D, et al. The association of endogenous sex hormones, adiposity, and insulin resistance with incident diabetes in postmenopausal women. J Clin Endocrinol Metab. 2009;94:4127–4135. doi: 10.1210/jc.2009-0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verchere CB, D’Alessio DA, Palmiter RD, Weir GC, Bonner-Weir S, et al. Islet amyloid formation associated with hyperglycemia in transgenic mice with pancreatic beta cell expression of human islet amyloid polypeptide. Proc Natl Acad Sci U S A. 1996;93:3492–3496. doi: 10.1073/pnas.93.8.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kahn SE, Andrikopoulos S, Verchere CB, Wang F, Hull RL, et al. Oophorectomy promotes islet amyloid formation in a transgenic mouse model of Type II diabetes. Diabetologia. 2000;43:1309–1312. doi: 10.1007/s001250051527. [DOI] [PubMed] [Google Scholar]
- 47.Geisler JG, Zawalich W, Zawalich K, Lakey JR, Stukenbrok H, et al. Estrogen can prevent or reverse obesity and diabetes in mice expressing human islet amyloid polypeptide. Diabetes. 2002;51:2158–2169. doi: 10.2337/diabetes.51.7.2158. [DOI] [PubMed] [Google Scholar]
- 48.Le May C, Chu K, Hu M, Ortega C, Simpson E, et al. Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci U S A. 2006;103:9232–9237. doi: 10.1073/pnas.0602956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alonso-Magdalena P, Ropero AB, Carrera MP, Cederroth CR, Baquie M, et al. Pancreatic insulin content regulation by the estrogen receptor ER alpha. PLoS ONE. 2008;3:e2069. doi: 10.1371/journal.pone.0002069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu S, Le May C, Wong WP, Ward RD, Clegg DJ, et al. Importance of extranuclear estrogen receptor-alpha and membrane G protein-coupled estrogen receptor in pancreatic islet survival. Diabetes. 2009;58:2292–2302. doi: 10.2337/db09-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu S, Mauvais-Jarvis F. Rapid, nongenomic estrogen actions protect pancreatic islet survival. Islets. 2009;1:273–275. doi: 10.4161/isl.1.3.9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martensson UE, Salehi SA, Windahl S, Gomez MF, Sward K, et al. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology. 2009;150:687–698. doi: 10.1210/en.2008-0623. [DOI] [PubMed] [Google Scholar]
- 53.Balhuizen A, Kumar R, Amisten S, Lundquist I, Salehi A. Activation of G protein-coupled receptor 30 modulates hormone secretion and counteracts cytokine-induced apoptosis in pancreatic islets of female mice. Mol Cell Endocrinol. 2010;320:16–24. doi: 10.1016/j.mce.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 54.Kang L, Zhang X, Xie Y, Tu Y, Wang D, et al. Involvement of estrogen receptor variant ER-alpha36, not GPR30, in nongenomic estrogen signaling. Mol Endocrinol. 2010;24:709–721. doi: 10.1210/me.2009-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soriano S, Ropero AB, Alonso-Magdalena P, Ripoll C, Quesada I, et al. Rapid regulation of K(ATP) channel activity by 17{beta}-estradiol in pancreatic {beta}-cells involves the estrogen receptor {beta} and the atrial natriuretic peptide receptor. Mol Endocrinol. 2009;23:1973–1982. doi: 10.1210/me.2009-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tokuyama Y, Sturis J, DePaoli AM, Takeda J, Stoffel M, et al. Evolution of beta-cell dysfunction in the male Zucker diabetic fatty rat. Diabetes. 1995;44:1447–1457. doi: 10.2337/diab.44.12.1447. [DOI] [PubMed] [Google Scholar]
- 57.Lee Y, Hirose H, Ohneda M, Johnson JH, McGarry JD, et al. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships. Proc Natl Acad Sci U S A. 1994;91:10878–10882. doi: 10.1073/pnas.91.23.10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tiano J, Le May C, Korach K, Mauvais-Jarvis F. The extranuclear estrogen receptora improves pancreatic islet lipid homeostasis. The Endocrine Society’s 92nd Annual Meeting; San Diego, CA. June 19–22, 2010; 2010. p. OR18-6. [Google Scholar]
- 59.Wong WP, Tiano JP, Liu S, Hewitt SC, Le May C, et al. Extranuclear estrogen receptor-{alpha} stimulates NeuroD1 binding to the insulin promoter and favors insulin synthesis. Proc Natl Acad Sci U S A. 2010;107:13057–62. doi: 10.1073/pnas.0914501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beato M. Gene regulation by steroid hormones. Cell. 1989;56:335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- 61.Schoenmakers E, Verrijdt G, Peeters B, Verhoeven G, Rombauts W, et al. Differences in DNA binding characteristics of the androgen and glucocorticoid receptors can determine hormone-specific responses. J Biol Chem. 2000;275:12290–12297. doi: 10.1074/jbc.275.16.12290. [DOI] [PubMed] [Google Scholar]
- 62.Foradori CD, Weiser MJ, Handa RJ. Non-genomic actions of androgens. Front Neuroendocrinol. 2008;29:169–181. doi: 10.1016/j.yfrne.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khaw KT, Barrett-Connor E. Lower endogenous androgens predict central adiposity in men. Ann Epidemiol. 1992;2:675–682. doi: 10.1016/1047-2797(92)90012-f. [DOI] [PubMed] [Google Scholar]
- 64.Zitzmann M, Faber S, Nieschlag E. Association of specific symptoms and metabolic risks with serum testosterone in older men. J Clin Endocrinol Metab. 2006;91:4335–4343. doi: 10.1210/jc.2006-0401. [DOI] [PubMed] [Google Scholar]
- 65.Bojesen A, Kristensen K, Birkebaek NH, Fedder J, Mosekilde L, et al. The metabolic syndrome is frequent in Klinefelter’s syndrome and is associated with abdominal obesity and hypogonadism. Diabetes Care. 2006;29:1591–1598. doi: 10.2337/dc06-0145. [DOI] [PubMed] [Google Scholar]
- 66.Basaria S, Muller DC, Carducci MA, Egan J, Dobs AS. Hyperglycemia and insulin resistance in men with prostate carcinoma who receive androgen-deprivation therapy. Cancer. 2006;106:581–588. doi: 10.1002/cncr.21642. [DOI] [PubMed] [Google Scholar]
- 67.Pitteloud N, Mootha VK, Dwyer AA, Hardin M, Lee H, et al. Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabetes Care. 2005;28:1636–1642. doi: 10.2337/diacare.28.7.1636. [DOI] [PubMed] [Google Scholar]
- 68.Moverare-Skrtic S, Venken K, Andersson N, Lindberg MK, Svensson J, et al. Dihydrotestosterone treatment results in obesity and altered lipid metabolism in orchidectomized mice. Obesity (Silver Spring) 2006;14:662–672. doi: 10.1038/oby.2006.75. [DOI] [PubMed] [Google Scholar]
- 69.Zitzmann M, Gromoll J, von Eckardstein A, Nieschlag E. The CAG repeat polymorphism in the androgen receptor gene modulates body fat mass and serum concentrations of leptin and insulin in men. Diabetologia. 2003;46:31–39. doi: 10.1007/s00125-002-0980-9. [DOI] [PubMed] [Google Scholar]
- 70.Fan W, Yanase T, Nomura M, Okabe T, Goto K, et al. Androgen receptor null male mice develop late-onset obesity caused by decreased energy expenditure and lipolytic activity but show normal insulin sensitivity with high adiponectin secretion. Diabetes. 2005;54:1000–1008. doi: 10.2337/diabetes.54.4.1000. [DOI] [PubMed] [Google Scholar]
- 71.Lin HY, Xu Q, Yeh S, Wang RS, Sparks JD, et al. Insulin and leptin resistance with hyperleptinemia in mice lacking androgen receptor. Diabetes. 2005;54:1717–1725. doi: 10.2337/diabetes.54.6.1717. [DOI] [PubMed] [Google Scholar]
- 72.Lanfranco F, Zitzmann M, Simoni M, Nieschlag E. Serum adiponectin levels in hypogonadal males: influence of testosterone replacement therapy. Clin Endocrinol (Oxf) 2004;60:500–507. doi: 10.1111/j.1365-2265.2004.02007.x. [DOI] [PubMed] [Google Scholar]
- 73.Nishizawa H, Shimomura I, Kishida K, Maeda N, Kuriyama H, et al. Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes. 2002;51:2734–2741. doi: 10.2337/diabetes.51.9.2734. [DOI] [PubMed] [Google Scholar]
- 74.Singh R, Artaza JN, Taylor WE, Gonzalez-Cadavid NF, Bhasin S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology. 2003;144:5081–5088. doi: 10.1210/en.2003-0741. [DOI] [PubMed] [Google Scholar]
- 75.Singh R, Artaza JN, Taylor WE, Braga M, Yuan X, et al. Testosterone inhibits adipogenic differentiation in 3T3-L1 cells: nuclear translocation of androgen receptor complex with beta-catenin and T-cell factor 4 may bypass canonical Wnt signaling to down-regulate adipogenic transcription factors. Endocrinology. 2006;147:141–154. doi: 10.1210/en.2004-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gentile MA, Nantermet PV, Vogel RL, Phillips R, Holder D, et al. Androgen-mediated improvement of body composition and muscle function involves a novel early transcriptional program including IGF1, mechano growth factor, and induction of {beta}-catenin. J Mol Endocrinol. 2010;44:55–73. doi: 10.1677/JME-09-0048. [DOI] [PubMed] [Google Scholar]
- 77.Fernando SM, Rao P, Niel L, Chatterjee D, Stagljar M, et al. Myocyte androgen receptors increase metabolic rate and improve body composition by reducing fat mass. Endocrinology. 2010;151:3125–3132. doi: 10.1210/en.2010-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu IC, Lin HY, Liu NC, Wang RS, Sparks JD, et al. Hyperleptinemia without obesity in male mice lacking androgen receptor in adipose tissue. Endocrinology. 2008;149:2361–2368. doi: 10.1210/en.2007-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 80.Fan W, Yanase T, Nishi Y, Chiba S, Okabe T, et al. Functional potentiation of leptin-signal transducer and activator of transcription 3 signaling by the androgen receptor. Endocrinology. 2008;149:6028–6036. doi: 10.1210/en.2008-0431. [DOI] [PubMed] [Google Scholar]
- 81.Maclaren NK, Neufeld M, McLaughlin JV, Taylor G. Androgen sensitization of streptozotocin-induced diabetes in mice. Diabetes. 1980;29:710–716. doi: 10.2337/diab.29.9.710. [DOI] [PubMed] [Google Scholar]
- 82.Paik SG, Michelis MA, Kim YT, Shin S. Induction of insulin-dependent diabetes by streptozotocin. Inhibition by estrogens and potentiation by androgens. Diabetes. 1982;31:724–729. doi: 10.2337/diab.31.8.724. [DOI] [PubMed] [Google Scholar]
- 83.Morimoto S, Mendoza-Rodriguez CA, Hiriart M, Larrieta ME, Vital P, et al. Protective effect of testosterone on early apoptotic damage induced by streptozotocin in rat pancreas. J Endocrinol. 2005;187:217–224. doi: 10.1677/joe.1.06357. [DOI] [PubMed] [Google Scholar]
- 84.Palomar-Morales M, Morimoto S, Mendoza-Rodriguez CA, Cerbon MA. The protective effect of testosterone on streptozotocin-induced apoptosis in beta cells is sex specific. Pancreas. 2010;39:193–200. doi: 10.1097/MPA.0b013e3181c156d9. [DOI] [PubMed] [Google Scholar]
- 85.Sato T, Matsumoto T, Yamada T, Watanabe T, Kawano H, et al. Late onset of obesity in male androgen receptor-deficient (AR KO) mice. Biochem Biophys Res Commun. 2003;300:167–171. doi: 10.1016/s0006-291x(02)02774-2. [DOI] [PubMed] [Google Scholar]
- 86.Dati E, Baroncelli GI, Mora S, Russo G, Baldinotti F, et al. Body composition and metabolic profile in women with complete androgen insensitivity syndrome. Sex Dev. 2009;3:188–193. doi: 10.1159/000228719. [DOI] [PubMed] [Google Scholar]
- 87.Achard C, Thiers J. Le virilisme pilaire et son association a l’insuffisance glycolytique (diabete des femmes a barbe) Bull Acad Natl Med Paris. 1921;86:51–55. [Google Scholar]
- 88.Coviello AD, Legro RS, Dunaif A. Adolescent girls with polycystic ovary syndrome have an increased risk of the metabolic syndrome associated with increasing androgen levels independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2006;91:492–497. doi: 10.1210/jc.2005-1666. [DOI] [PubMed] [Google Scholar]
- 89.Diamond MP, Grainger D, Diamond MC, Sherwin RS, Defronzo RA. Effects of methyltestosterone on insulin secretion and sensitivity in women. J Clin Endocrinol Metab. 1998;83:4420–4425. doi: 10.1210/jcem.83.12.5333. [DOI] [PubMed] [Google Scholar]
- 90.Holmang A, Svedberg J, Jennische E, Bjorntorp P. Effects of testosterone on muscle insulin sensitivity and morphology in female rats. Am J Physiol. 1990;259:E555–560. doi: 10.1152/ajpendo.1990.259.4.E555. [DOI] [PubMed] [Google Scholar]
- 91.O’Meara NM, Blackman JD, Ehrmann DA, Barnes RB, Jaspan JB, et al. Defects in beta-cell function in functional ovarian hyperandrogenism. J Clin Endocrinol Metab. 1993;76:1241–1247. doi: 10.1210/jcem.76.5.8496316. [DOI] [PubMed] [Google Scholar]
- 92.Holte J, Bergh T, Berne C, Berglund L, Lithell H. Enhanced early insulin response to glucose in relation to insulin resistance in women with polycystic ovary syndrome and normal glucose tolerance. J Clin Endocrinol Metab. 1994;78:1052–1058. doi: 10.1210/jcem.78.5.8175959. [DOI] [PubMed] [Google Scholar]
- 93.Goodarzi MO, Erickson S, Port SC, Jennrich RI, Korenman SG. beta-Cell function: a key pathological determinant in polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:310–315. doi: 10.1210/jc.2004-1006. [DOI] [PubMed] [Google Scholar]
- 94.Liu S, Navarro G, Mauvais-Jarvis F. Androgen excess produces systemic oxidative stress and predisposes to beta-cell failure in female mice. PLoS One. 2010;5:e11302. doi: 10.1371/journal.pone.0011302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gonzalez F, Rote NS, Minium J, Kirwan JP. Reactive oxygen species-induced oxidative stress in the development of insulin resistance and hyperandrogenism in polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:336–340. doi: 10.1210/jc.2005-1696. [DOI] [PubMed] [Google Scholar]
- 96.Navarro G, Liu S, De Gendt K, Verhoeven G, Mauvais-Jarvis F. Androgen excess in females predisposes to insulin deficiency via AR in beta-cells. The Endocrine Society’s 92nd Annual Meeting; San Diego, CA. June 19–22, 2010; 2010. p. PI-468. [Google Scholar]
- 97.Corbould A. Effects of androgens on insulin action in women: is androgen excess a component of female metabolic syndrome? Diabetes Metab Res Rev. 2008;24:520–532. doi: 10.1002/dmrr.872. [DOI] [PubMed] [Google Scholar]