Figure 4. Comparing Interactions in Alternate Kv7 Subtypes.
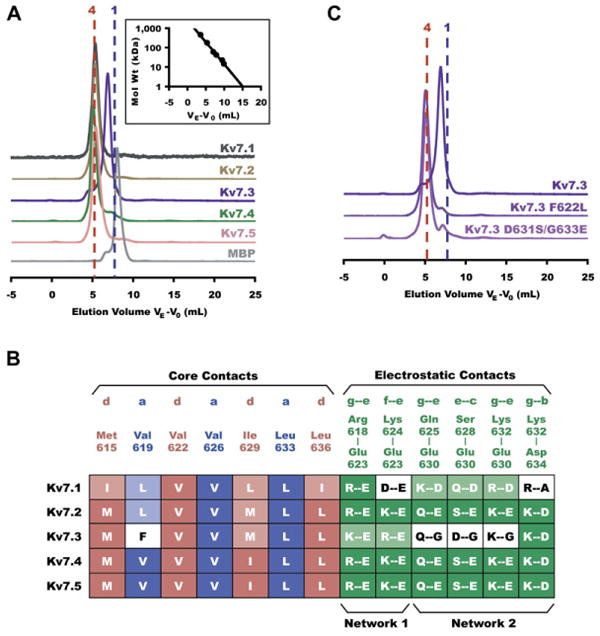
(A) Stoichiometry of coiled-coil assembly domains in all five Kv7 subtypes shown by Superdex200 (Amersham Biosciences) size exclusion chromatography. Normalized absorbance is plotted against elution volume VE corrected for void elution volume V0 as in Figure 3B. All samples were loaded at a concentration of 50 μM. Vertically displaced chromatograms show traces for, from top to bottom, Kv7.1 (black), Kv7.2 (orange), Kv7.3 (purple), Kv7.4 (green), Kv7.5 (pink), and MBP (gray). Vertical dotted lines indicate the predicted elution volumes of tetrameric (red) and monomeric (blue) fusion proteins. (Inset) Standard curve used to calculate molecular weight of eluted proteins on the Superdex200 column. Molecular weights for each are as follows (observed ± SD, expected monomer, expected tetramer); Kv7.1 (180 ± 2 kD, 49.4 kD, 198 kD); Kv7.2 (203 ± 6 kD, 49.3 kD, 197 kD); Kv7.3 (90.3 ± 2 kD, 49.9 kD, 200 kD); Kv7.4 (207 ± 6 kD, 48.8 kD, 195 kD); Kv7.5 (191 ± 6 kD, 48.9 kD, 196 kD).
(B) Comparative interaction mapping in all subtypes. Column labels identify residue types involved in hydrophobic “a” (blue) and “d” (pink) layer contacts and electrostatic interactions (green) observed in the Kv7.4 coiled-coil structure. Filled boxes in table indicate entirely conserved interactions; shaded boxes indicate nonconserved residues that are still capable of interacting as predicted; white boxes indicate unfavorable contacts. Electrostatic interactions involved in networks 1 and 2 are indicated below the alignment.
(C) Stoichiometry of mutant coiled-coil assembly domains as determined by size exclusion. Kv7.3 A-domain Tail mutants F622L and D631S/G633E restore tetramerization. Molecular weights for each are as follows (observed, expected monomer, expected tetramer); Kv7.3 (90.3 kD, 49.9 kD, 200 kD); Kv7.3 F622L (212 kD, 49.9 kD, 200 kD); Kv7.3 D631S/G633E (208 kD, 49.9 kD, 200 kD). All samples were loaded onto the column at a concentration of 50 μM.
