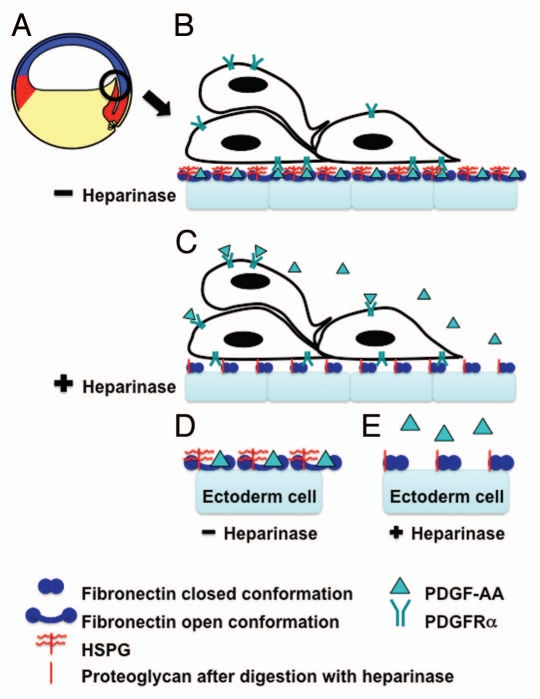
Figure 1.
Heparan sulfate modification of fibronectin enables PDGF-AA-fibronectin interactions necessary for directed cell migration during Xenopus gastrulation. (A) A schematic of a Xenopus embryo immediately after the onset of gastrulation (stage 10+). The prospective mesoderm (red), ectoderm (blue) and endoderm (yellow) are indicated. The black circle indicates migrating anterior mesendoderm cells and the region of tissue shown in (B and C). (B) In vivo the inner surface of the ectoderm (blue blocks) is lined with a fibronectin-rich ECM. This fibronectin is proposed to be in its open conformation (blue dumbbells) due to the presence of heparan sulfate proteoglycans on the surface of these cells (indicated in red). PDGF (green triangle) secreted by the ectoderm cells binds to the C-terminus of the fibronectin. This PDGF is then available for PDGFRα (green fork) binding and activation. The PDGFRα is expressed on the migrating mesendoderm cells. This ligand receptor interaction guides the directed movement of these cells. (C) Heparinase III treatment of these tissues during matrix deposition digests the heparan sulfate side chains leaving the proteoglycan core (red line), which does not alter fibronectin structure leaving it in the closed conformation. PDGF secreted by the ectoderm cells can then diffuse from the site of secretion leaving it available to bind PDGFRαs over the entire surface of the mesendoderm cells and thus, disrupting directed movement. (D and E) A schematic of a “higher magnification” view of one cell in (B and C). A key of the symbols used in (B–D) is shown.