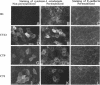
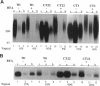
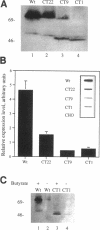
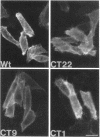
Abstract
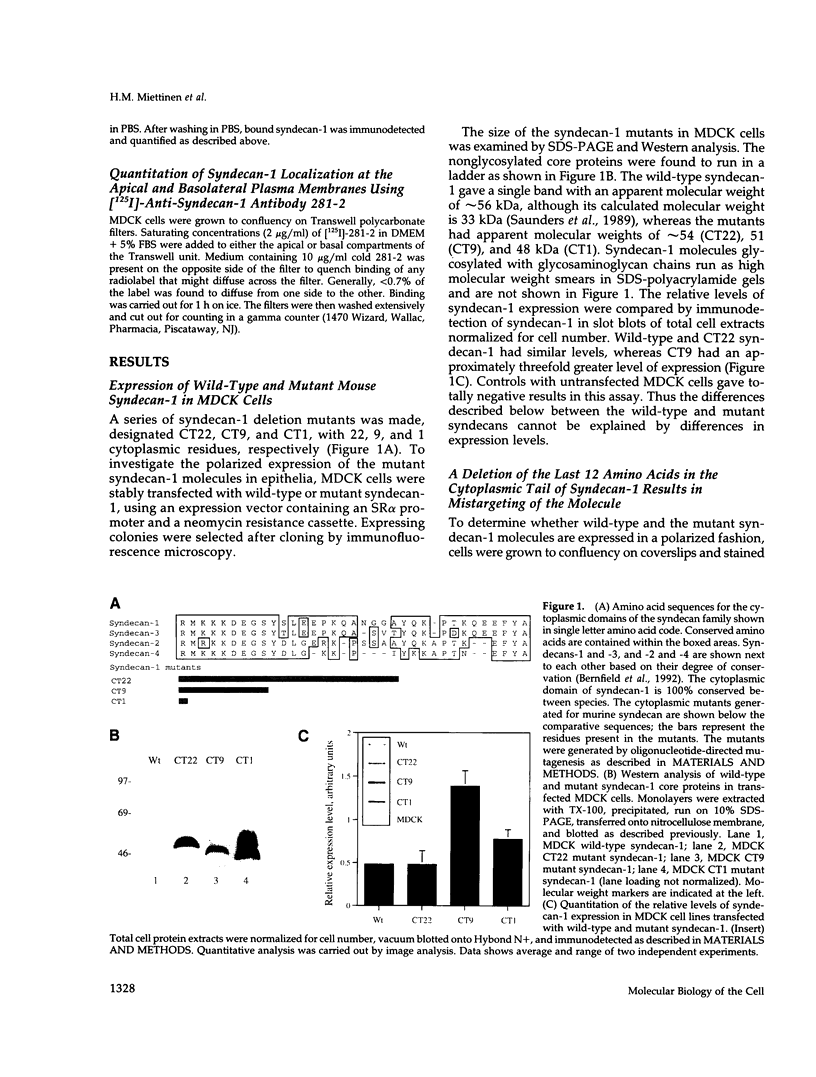
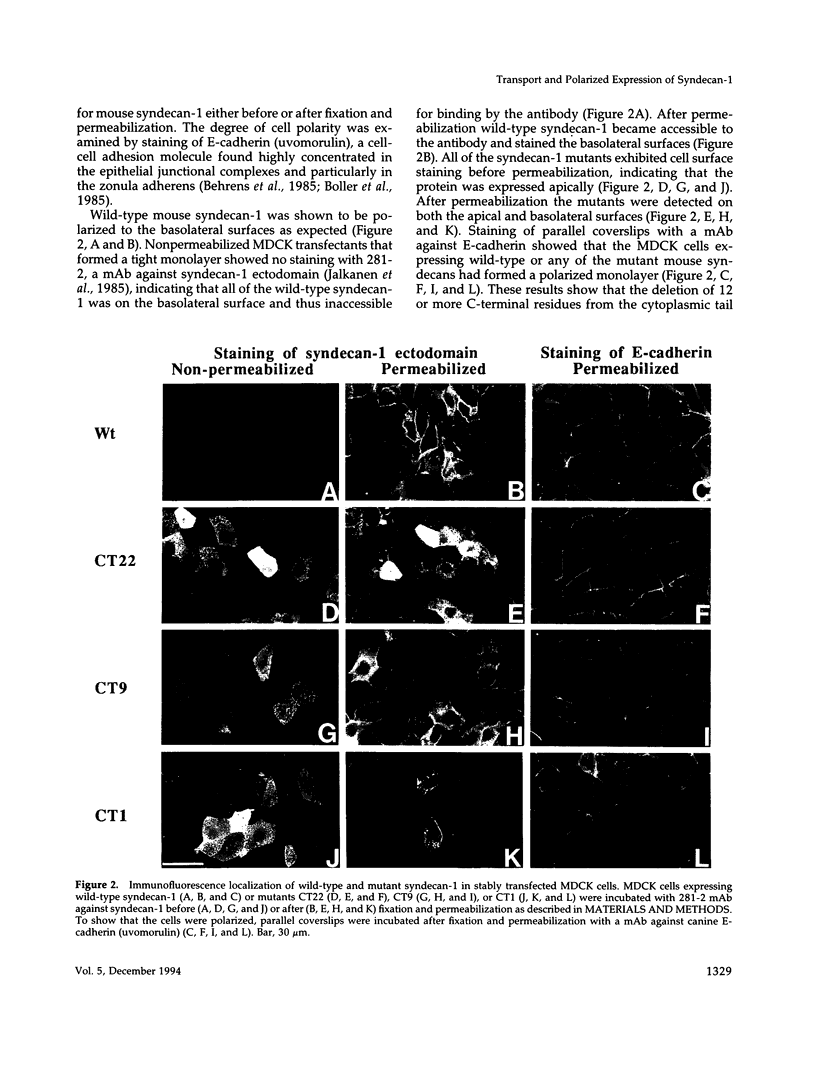
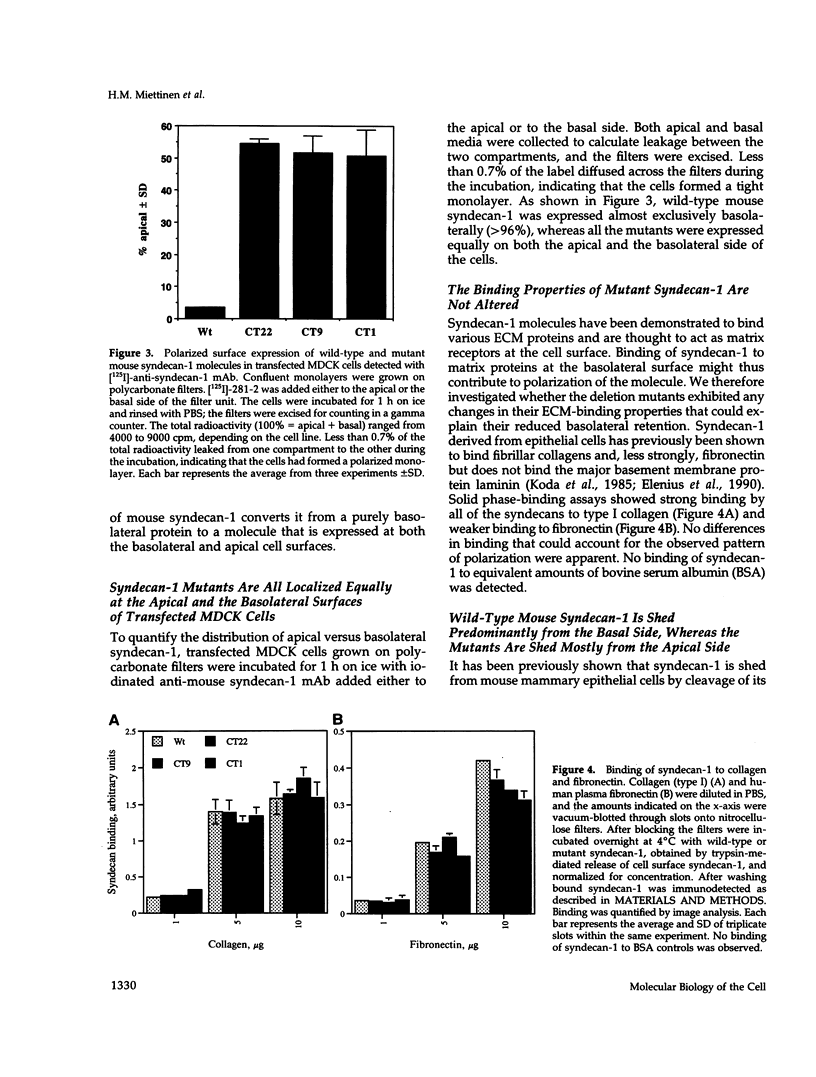
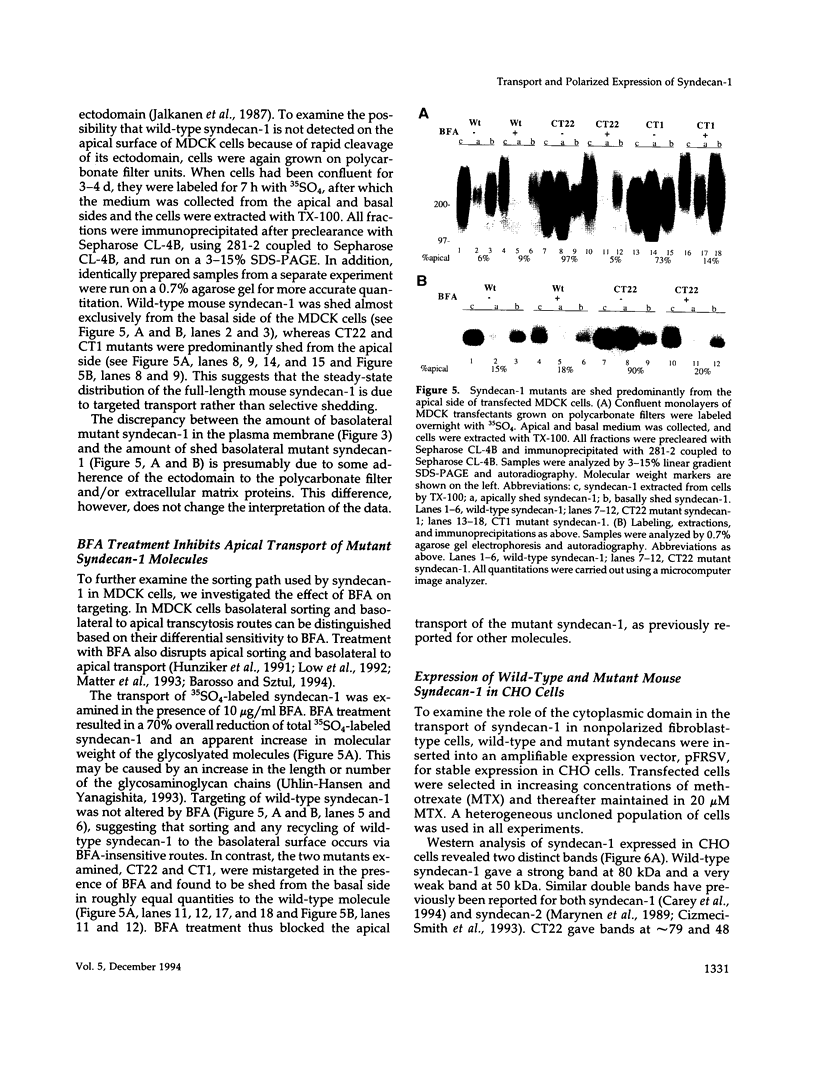
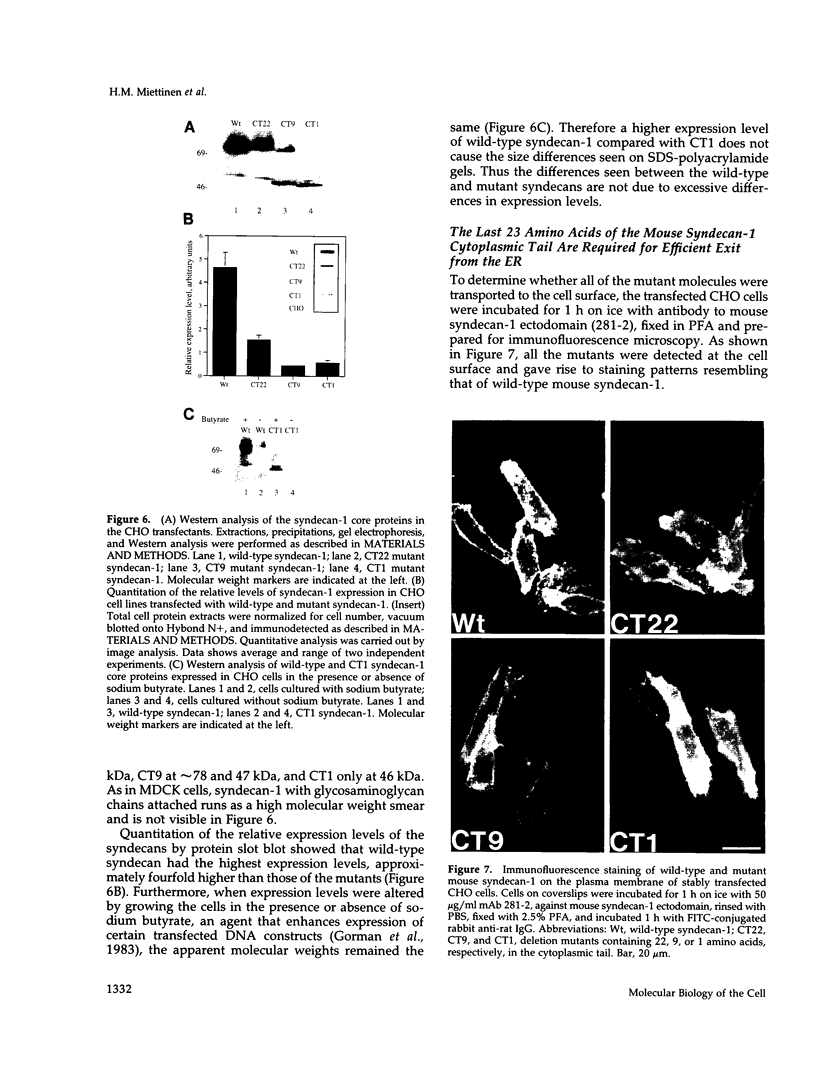
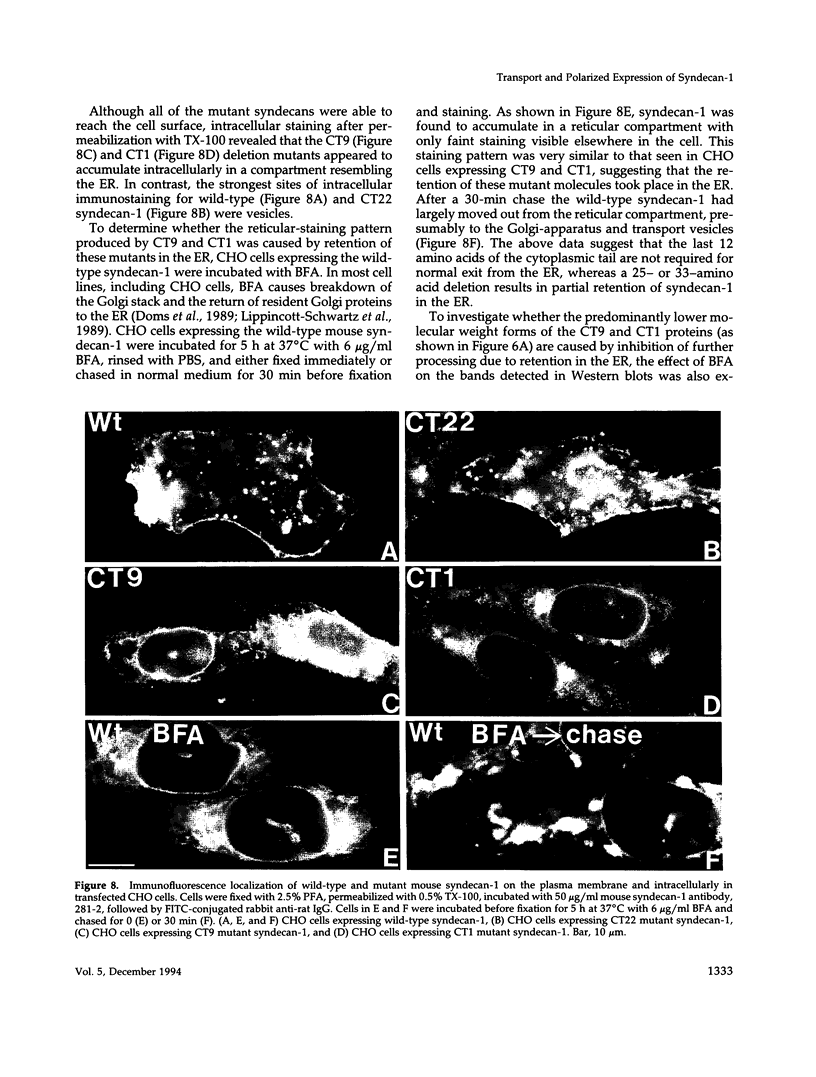
Madin-Darby canine kidney (MDCK) cells and Chinese hamster ovary (CHO) cells were transfected with wild-type and cytoplasmic deletion mutants of mouse syndecan-1 to study the requirements for transport and polarized expression of this proteoglycan. Expression in MDCK cells revealed that wild-type syndecan-1 is directed to the basolateral surface via a brefeldin A-insensitive route. A deletion of the last 12 amino acids of the syndecan-1 cytoplasmic tail (CT22) was sufficient to result in the appearance of mutant proteoglycans at both the basolateral and apical cell surfaces. Treatment with brefeldin A was able to prevent apical transport of the mutants. We thus propose that the C-terminal part of the cytoplasmic tail is required for steady-state basolateral distribution of syndecan-1. In CHO cells a deletion of the last 25 or 33 amino acids of the 34-residue cytoplasmic domain (CT9 and CT1, respectively) resulted in partial retention of the mutants in the endoplasmic reticulum (ER). A deletion mutant lacking the last 12 amino acids (CT22) was not retained. Interestingly, the unglycosylated core proteins of the CT9 and CT1 mutants showed a significantly lower apparent molecular weight when analyzed by sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis than wild-type syndecan-1. However, when CHO transfectants expressing the CT1 mutant were incubated with brefeldin A, causing fusion of the ER and Golgi, CT1 ran with an almost equally high apparent molecular weight as the wild-type molecule. This would suggest that syndecan-1 undergoes extensive posttranslational modifications or forms an SDS-resistant dimer/complex after transit from the ER.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apodaca G., Aroeti B., Tang K., Mostov K. E. Brefeldin-A inhibits the delivery of the polymeric immunoglobulin receptor to the basolateral surface of MDCK cells. J Biol Chem. 1993 Sep 25;268(27):20380–20385. [PubMed] [Google Scholar]
- Aroeti B., Mostov K. E. Polarized sorting of the polymeric immunoglobulin receptor in the exocytotic and endocytotic pathways is controlled by the same amino acids. EMBO J. 1994 May 15;13(10):2297–2304. doi: 10.1002/j.1460-2075.1994.tb06513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso M., Sztul E. S. Basolateral to apical transcytosis in polarized cells is indirect and involves BFA and trimeric G protein sensitive passage through the apical endosome. J Cell Biol. 1994 Jan;124(1-2):83–100. doi: 10.1083/jcb.124.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J., Birchmeier W., Goodman S. L., Imhof B. A. Dissociation of Madin-Darby canine kidney epithelial cells by the monoclonal antibody anti-arc-1: mechanistic aspects and identification of the antigen as a component related to uvomorulin. J Cell Biol. 1985 Oct;101(4):1307–1315. doi: 10.1083/jcb.101.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernfield M., Kokenyesi R., Kato M., Hinkes M. T., Spring J., Gallo R. L., Lose E. J. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu Rev Cell Biol. 1992;8:365–393. doi: 10.1146/annurev.cb.08.110192.002053. [DOI] [PubMed] [Google Scholar]
- Boller K., Vestweber D., Kemler R. Cell-adhesion molecule uvomorulin is localized in the intermediate junctions of adult intestinal epithelial cells. J Cell Biol. 1985 Jan;100(1):327–332. doi: 10.1083/jcb.100.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer C. B., Roth M. G. A single amino acid change in the cytoplasmic domain alters the polarized delivery of influenza virus hemagglutinin. J Cell Biol. 1991 Aug;114(3):413–421. doi: 10.1083/jcb.114.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp R. L., Kraus T. A., Puré E. Variations in the cytoskeletal interaction and posttranslational modification of the CD44 homing receptor in macrophages. J Cell Biol. 1991 Dec;115(5):1283–1292. doi: 10.1083/jcb.115.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey D. J., Evans D. M., Stahl R. C., Asundi V. K., Conner K. J., Garbes P., Cizmeci-Smith G. Molecular cloning and characterization of N-syndecan, a novel transmembrane heparan sulfate proteoglycan. J Cell Biol. 1992 Apr;117(1):191–201. doi: 10.1083/jcb.117.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey D. J., Stahl R. C., Cizmeci-Smith G., Asundi V. K. Syndecan-1 expressed in Schwann cells causes morphological transformation and cytoskeletal reorganization and associates with actin during cell spreading. J Cell Biol. 1994 Jan;124(1-2):161–170. doi: 10.1083/jcb.124.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova J. E., Apodaca G., Mostov K. E. An autonomous signal for basolateral sorting in the cytoplasmic domain of the polymeric immunoglobulin receptor. Cell. 1991 Jul 12;66(1):65–75. doi: 10.1016/0092-8674(91)90139-p. [DOI] [PubMed] [Google Scholar]
- Cizmeci-Smith G., Asundi V., Stahl R. C., Teichman L. J., Chernousov M., Cowan K., Carey D. J. Regulated expression of syndecan in vascular smooth muscle cells and cloning of rat syndecan core protein cDNA. J Biol Chem. 1992 Aug 5;267(22):15729–15736. [PubMed] [Google Scholar]
- Cizmeci-Smith G., Stahl R. C., Showalter L. J., Carey D. J. Differential expression of transmembrane proteoglycans in vascular smooth muscle cells. J Biol Chem. 1993 Sep 5;268(25):18740–18747. [PubMed] [Google Scholar]
- David G., van der Schueren B., Marynen P., Cassiman J. J., van den Berghe H. Molecular cloning of amphiglycan, a novel integral membrane heparan sulfate proteoglycan expressed by epithelial and fibroblastic cells. J Cell Biol. 1992 Aug;118(4):961–969. doi: 10.1083/jcb.118.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doms R. W., Russ G., Yewdell J. W. Brefeldin A redistributes resident and itinerant Golgi proteins to the endoplasmic reticulum. J Cell Biol. 1989 Jul;109(1):61–72. doi: 10.1083/jcb.109.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti C. G., Kartenbeck J., Simons K. Polarized distribution of the viral glycoproteins of vesicular stomatitis, fowl plague and Semliki Forest viruses in hippocampal neurons in culture: a light and electron microscopy study. Brain Res. 1993 Apr 30;610(1):141–147. doi: 10.1016/0006-8993(93)91227-j. [DOI] [PubMed] [Google Scholar]
- Dotti C. G., Simons K. Polarized sorting of viral glycoproteins to the axon and dendrites of hippocampal neurons in culture. Cell. 1990 Jul 13;62(1):63–72. doi: 10.1016/0092-8674(90)90240-f. [DOI] [PubMed] [Google Scholar]
- Elenius K., Salmivirta M., Inki P., Mali M., Jalkanen M. Binding of human syndecan to extracellular matrix proteins. J Biol Chem. 1990 Oct 15;265(29):17837–17843. [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Howard B. H., Reeves R. Expression of recombinant plasmids in mammalian cells is enhanced by sodium butyrate. Nucleic Acids Res. 1983 Nov 11;11(21):7631–7648. doi: 10.1093/nar/11.21.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerton R. W., Krzeminski K. A., Mays R. W., Ryan T. A., Wollner D. A., Nelson W. J. Mechanism for regulating cell surface distribution of Na+,K(+)-ATPase in polarized epithelial cells. Science. 1991 Nov 8;254(5033):847–850. doi: 10.1126/science.1658934. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Hayashi M., Jalkanen M., Firestone J. H., Trelstad R. L., Bernfield M. Immunocytochemistry of cell surface heparan sulfate proteoglycan in mouse tissues. A light and electron microscopic study. J Histochem Cytochem. 1987 Oct;35(10):1079–1088. doi: 10.1177/35.10.2957423. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Sommarin Y., Hedbom E., Wieslander J., Larsson B. Assay of proteoglycan populations using agarose-polyacrylamide gel electrophoresis. Anal Biochem. 1985 Nov 15;151(1):41–48. doi: 10.1016/0003-2697(85)90050-8. [DOI] [PubMed] [Google Scholar]
- Horwich A. L., Fenton W. A., Firgaira F. A., Fox J. E., Kolansky D., Mellman I. S., Rosenberg L. E. Expression of amplified DNA sequences for ornithine transcarbamylase in HeLa cells: arginine residues may be required for mitochondrial import of enzyme precursor. J Cell Biol. 1985 May;100(5):1515–1521. doi: 10.1083/jcb.100.5.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W., Fumey C. A di-leucine motif mediates endocytosis and basolateral sorting of macrophage IgG Fc receptors in MDCK cells. EMBO J. 1994 Jul 1;13(13):2963–2969. doi: 10.1002/j.1460-2075.1994.tb06594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W., Whitney J. A., Mellman I. Selective inhibition of transcytosis by brefeldin A in MDCK cells. Cell. 1991 Nov 1;67(3):617–627. doi: 10.1016/0092-8674(91)90535-7. [DOI] [PubMed] [Google Scholar]
- Jalkanen M., Nguyen H., Rapraeger A., Kurn N., Bernfield M. Heparan sulfate proteoglycans from mouse mammary epithelial cells: localization on the cell surface with a monoclonal antibody. J Cell Biol. 1985 Sep;101(3):976–984. doi: 10.1083/jcb.101.3.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkanen M., Rapraeger A., Saunders S., Bernfield M. Cell surface proteoglycan of mouse mammary epithelial cells is shed by cleavage of its matrix-binding ectodomain from its membrane-associated domain. J Cell Biol. 1987 Dec;105(6 Pt 2):3087–3096. doi: 10.1083/jcb.105.6.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer M. C., Stephans J. C., Crawford K., Okino K., Barr P. J. Ligand-affinity cloning and structure of a cell surface heparan sulfate proteoglycan that binds basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6985–6989. doi: 10.1073/pnas.87.18.6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koda J. E., Rapraeger A., Bernfield M. Heparan sulfate proteoglycans from mouse mammary epithelial cells. Cell surface proteoglycan as a receptor for interstitial collagens. J Biol Chem. 1985 Jul 5;260(13):8157–8162. [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lacy B. E., Underhill C. B. The hyaluronate receptor is associated with actin filaments. J Cell Biol. 1987 Sep;105(3):1395–1404. doi: 10.1083/jcb.105.3.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan L. C., Bonifacino J. S., Klausner R. D. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989 Mar 10;56(5):801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan L., Tipper C., Amherdt M., Orci L., Klausner R. D. Brefeldin A's effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell. 1991 Nov 1;67(3):601–616. doi: 10.1016/0092-8674(91)90534-6. [DOI] [PubMed] [Google Scholar]
- Lories V., Cassiman J. J., Van den Berghe H., David G. Differential expression of cell surface heparan sulfate proteoglycans in human mammary epithelial cells and lung fibroblasts. J Biol Chem. 1992 Jan 15;267(2):1116–1122. [PubMed] [Google Scholar]
- Low S. H., Tang B. L., Wong S. H., Hong W. Selective inhibition of protein targeting to the apical domain of MDCK cells by brefeldin A. J Cell Biol. 1992 Jul;118(1):51–62. doi: 10.1083/jcb.118.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K., Mellman I. Mechanisms of cell polarity: sorting and transport in epithelial cells. Curr Opin Cell Biol. 1994 Aug;6(4):545–554. doi: 10.1016/0955-0674(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Matter K., Whitney J. A., Yamamoto E. M., Mellman I. Common signals control low density lipoprotein receptor sorting in endosomes and the Golgi complex of MDCK cells. Cell. 1993 Sep 24;74(6):1053–1064. doi: 10.1016/0092-8674(93)90727-8. [DOI] [PubMed] [Google Scholar]
- Matter K., Yamamoto E. M., Mellman I. Structural requirements and sequence motifs for polarized sorting and endocytosis of LDL and Fc receptors in MDCK cells. J Cell Biol. 1994 Aug;126(4):991–1004. doi: 10.1083/jcb.126.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen H. M., Jalkanen M. The cytoplasmic domain of syndecan-1 is not required for association with Triton X-100-insoluble material. J Cell Sci. 1994 Jun;107(Pt 6):1571–1581. doi: 10.1242/jcs.107.6.1571. [DOI] [PubMed] [Google Scholar]
- Miettinen H. M., Matter K., Hunziker W., Rose J. K., Mellman I. Fc receptor endocytosis is controlled by a cytoplasmic domain determinant that actively prevents coated pit localization. J Cell Biol. 1992 Feb;116(4):875–888. doi: 10.1083/jcb.116.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen H. M., Rose J. K., Mellman I. Fc receptor isoforms exhibit distinct abilities for coated pit localization as a result of cytoplasmic domain heterogeneity. Cell. 1989 Jul 28;58(2):317–327. doi: 10.1016/0092-8674(89)90846-5. [DOI] [PubMed] [Google Scholar]
- Misumi Y., Misumi Y., Miki K., Takatsuki A., Tamura G., Ikehara Y. Novel blockade by brefeldin A of intracellular transport of secretory proteins in cultured rat hepatocytes. J Biol Chem. 1986 Aug 25;261(24):11398–11403. [PubMed] [Google Scholar]
- Mostov K., Apodaca G., Aroeti B., Okamoto C. Plasma membrane protein sorting in polarized epithelial cells. J Cell Biol. 1992 Feb;116(3):577–583. doi: 10.1083/jcb.116.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naim H. Y., Roth M. G. Characteristics of the internalization signal in the Y543 influenza virus hemagglutinin suggest a model for recognition of internalization signals containing tyrosine. J Biol Chem. 1994 Feb 11;269(6):3928–3933. [PubMed] [Google Scholar]
- Neame S. J., Isacke C. M. The cytoplasmic tail of CD44 is required for basolateral localization in epithelial MDCK cells but does not mediate association with the detergent-insoluble cytoskeleton of fibroblasts. J Cell Biol. 1993 Jun;121(6):1299–1310. doi: 10.1083/jcb.121.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J., Shore E. M., Wang A. Z., Hammerton R. W. Identification of a membrane-cytoskeletal complex containing the cell adhesion molecule uvomorulin (E-cadherin), ankyrin, and fodrin in Madin-Darby canine kidney epithelial cells. J Cell Biol. 1990 Feb;110(2):349–357. doi: 10.1083/jcb.110.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce A., Lyon M., Hampson I. N., Cowling G. J., Gallagher J. T. Molecular cloning of the major cell surface heparan sulfate proteoglycan from rat liver. J Biol Chem. 1992 Feb 25;267(6):3894–3900. [PubMed] [Google Scholar]
- Prill V., Lehmann L., von Figura K., Peters C. The cytoplasmic tail of lysosomal acid phosphatase contains overlapping but distinct signals for basolateral sorting and rapid internalization in polarized MDCK cells. EMBO J. 1993 May;12(5):2181–2193. doi: 10.1002/j.1460-2075.1993.tb05866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prydz K., Hansen S. H., Sandvig K., van Deurs B. Effects of brefeldin A on endocytosis, transcytosis and transport to the Golgi complex in polarized MDCK cells. J Cell Biol. 1992 Oct;119(2):259–272. doi: 10.1083/jcb.119.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapraeger A. C., Krufka A., Olwin B. B. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991 Jun 21;252(5013):1705–1708. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]
- Rapraeger A. C. The coordinated regulation of heparan sulfate, syndecans and cell behavior. Curr Opin Cell Biol. 1993 Oct;5(5):844–853. doi: 10.1016/0955-0674(93)90034-n. [DOI] [PubMed] [Google Scholar]
- Rapraeger A., Jalkanen M., Bernfield M. Cell surface proteoglycan associates with the cytoskeleton at the basolateral cell surface of mouse mammary epithelial cells. J Cell Biol. 1986 Dec;103(6 Pt 2):2683–2696. doi: 10.1083/jcb.103.6.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapraeger A., Jalkanen M., Endo E., Koda J., Bernfield M. The cell surface proteoglycan from mouse mammary epithelial cells bears chondroitin sulfate and heparan sulfate glycosaminoglycans. J Biol Chem. 1985 Sep 15;260(20):11046–11052. [PubMed] [Google Scholar]
- Rose J. K., Bergmann J. E. Altered cytoplasmic domains affect intracellular transport of the vesicular stomatitis virus glycoprotein. Cell. 1983 Sep;34(2):513–524. doi: 10.1016/0092-8674(83)90384-7. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Buonocore L., Whitt M. A. A new cationic liposome reagent mediating nearly quantitative transfection of animal cells. Biotechniques. 1991 Apr;10(4):520–525. [PubMed] [Google Scholar]
- Salmivirta M., Elenius K., Vainio S., Hofer U., Chiquet-Ehrismann R., Thesleff I., Jalkanen M. Syndecan from embryonic tooth mesenchyme binds tenascin. J Biol Chem. 1991 Apr 25;266(12):7733–7739. [PubMed] [Google Scholar]
- Sandvig K., Prydz K., Hansen S. H., van Deurs B. Ricin transport in brefeldin A-treated cells: correlation between Golgi structure and toxic effect. J Cell Biol. 1991 Nov;115(4):971–981. doi: 10.1083/jcb.115.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders S., Bernfield M. Cell surface proteoglycan binds mouse mammary epithelial cells to fibronectin and behaves as a receptor for interstitial matrix. J Cell Biol. 1988 Feb;106(2):423–430. doi: 10.1083/jcb.106.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders S., Jalkanen M., O'Farrell S., Bernfield M. Molecular cloning of syndecan, an integral membrane proteoglycan. J Cell Biol. 1989 Apr;108(4):1547–1556. doi: 10.1083/jcb.108.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen C. C., Levinson A. D. Isolation and expression of an altered mouse dihydrofolate reductase cDNA. Proc Natl Acad Sci U S A. 1983 May;80(9):2495–2499. doi: 10.1073/pnas.80.9.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Mosher D. F., Rapraeger A. Heparan sulfate-mediated binding of epithelial cell surface proteoglycan to thrombospondin. J Biol Chem. 1989 Feb 15;264(5):2885–2889. [PubMed] [Google Scholar]
- Takebe Y., Seiki M., Fujisawa J., Hoy P., Yokota K., Arai K., Yoshida M., Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988 Jan;8(1):466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarone G., Ferracini R., Galetto G., Comoglio P. A cell surface integral membrane glycoprotein of 85,000 mol wt (gp85) associated with triton X-100-insoluble cell skeleton. J Cell Biol. 1984 Aug;99(2):512–519. doi: 10.1083/jcb.99.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. C., Brewer C. B., Roth M. G. Vesicular stomatitis virus glycoprotein contains a dominant cytoplasmic basolateral sorting signal critically dependent upon a tyrosine. J Biol Chem. 1993 Feb 15;268(5):3313–3320. [PubMed] [Google Scholar]
- Thomas D. C., Roth M. G. The basolateral targeting signal in the cytoplasmic domain of glycoprotein G from vesicular stomatitis virus resembles a variety of intracellular targeting motifs related by primary sequence but having diverse targeting activities. J Biol Chem. 1994 Jun 3;269(22):15732–15739. [PubMed] [Google Scholar]
- Thornton D. J., Nieduszynski I. A., Oates K., Sheehan J. K. Electron-microscopic and electrophoretic studies of bovine femoral-head cartilage proteoglycan fractions. Biochem J. 1986 Nov 15;240(1):41–48. doi: 10.1042/bj2400041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlin-Hansen L., Yanagishita M. Differential effect of brefeldin A on the biosynthesis of heparan sulfate and chondroitin/dermatan sulfate proteoglycans in rat ovarian granulosa cells in culture. J Biol Chem. 1993 Aug 15;268(23):17370–17376. [PubMed] [Google Scholar]
- Wagner M., Rajasekaran A. K., Hanzel D. K., Mayor S., Rodriguez-Boulan E. Brefeldin A causes structural and functional alterations of the trans-Golgi network of MDCK cells. J Cell Sci. 1994 Apr;107(Pt 4):933–943. doi: 10.1242/jcs.107.4.933. [DOI] [PubMed] [Google Scholar]
- Wills J. W., Srinivas R. V., Hunter E. Mutations of the Rous sarcoma virus env gene that affect the transport and subcellular location of the glycoprotein products. J Cell Biol. 1984 Dec;99(6):2011–2023. doi: 10.1083/jcb.99.6.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A., Couchman J. R. Syndecan 4 heparan sulfate proteoglycan is a selectively enriched and widespread focal adhesion component. Mol Biol Cell. 1994 Feb;5(2):183–192. doi: 10.1091/mbc.5.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yayon A., Klagsbrun M., Esko J. D., Leder P., Ornitz D. M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991 Feb 22;64(4):841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis using M13-derived vectors: an efficient and general procedure for the production of point mutations in any fragment of DNA. Nucleic Acids Res. 1982 Oct 25;10(20):6487–6500. doi: 10.1093/nar/10.20.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuniga M. C., Hood L. E. Clonal variation in cell surface display of an H-2 protein lacking a cytoplasmic tail. J Cell Biol. 1986 Jan;102(1):1–10. doi: 10.1083/jcb.102.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]