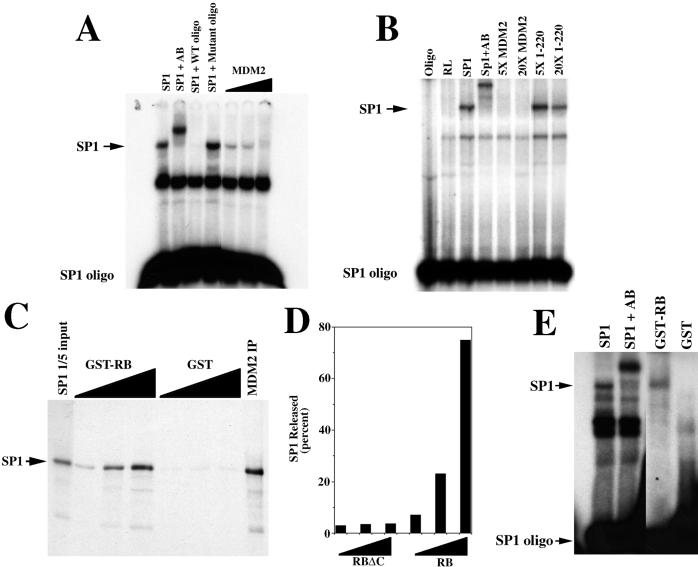
Figure 4.
MDM2 inhibits the DNA-binding activity of Sp1, and pRB releases Sp1 from MDM2. (A) Gel mobility-shift assay showing that in vitro-translated MDM2 inhibits the DNA-binding activity of in vitro-translated Sp1. Control lanes show that adding an Sp1-specific antibody (AB) to the reaction results in a supershift of the Sp1-dependent band, and that wild-type (WT) but not mutant oligonucleotide (oligo) interferes with the same Sp1-dependent band. (B) Gel mobility shift assay showing that 5- (5×) and 20- (20×) fold excess of the N-terminal mutant (1–220) of MDM2 does not interfere with the DNA-binding activity of in vitro-translated Sp1. Control lanes show that adding an Sp1-specific antibody (AB) to the reaction results in a supershift of the Sp1-dependent band. The amount of Sp1, full-length MDM2, and the N-terminal mutant of MDM2 (1–220) was determined by 35S incorporation. (C) pRB releases Sp1 from MDM2. In vitro-translated and radiolabeled Sp1 was immunoprecipitated with in vitro-translated MDM2 (MDM2 IP). The MDM2-Sp1 immunoprecipitate was incubated with increasing amounts of either GST-RB or GST alone and re-immunoprecipitated, and proteins in the supernatant were separated by gel electrophoresis. (D) The C terminus of pRB is required for Sp1 release. In vitro-translated and radiolabeled Sp1 was immunoprecipitated with in vitro-translated MDM2, as in C above. The MDM2-Sp1 immunoprecipitate was incubated with increasing amounts (25, 125, and 500 ng) of either GST-RB (379–928) or GSTRBΔC (379–824) and re-immunoprecipitated. Released Sp1 was quantitated by using a PhosphorImager after SDS/PAGE. Values represent the amount of Sp1 released, expressed as the percentage of Sp1 immunoprecipitated by MDM2. (E) Released Sp1 can bind DNA. In vitro-translated Sp1 was incubated with in vitro-translated MDM2 and immunoprecipitated and released as in C above, except that the released Sp1 was subjected to gel mobility shift analysis. A control lane shows a supershift of the Sp1 band with the Sp1-specific antibody.