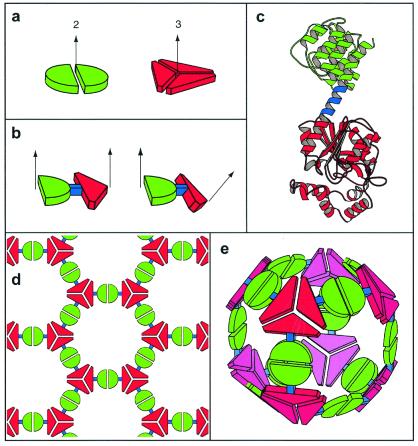
Figure 1.
A general strategy for designing fusion proteins that assemble into symmetric nanostructures. (a) The green semicircle represents a natural dimeric protein (i.e., a protein that associates with one other copy of itself), whereas the red shape represents a trimeric protein. The symmetry axes of the natural oligomers are shown. (b) The two natural proteins are combined by genetic methods into a single fusion protein. Each of the original natural proteins serves as an “oligomerization domain” in the designed fusion protein. Two different hypothetical fusion proteins are shown to illustrate that the oligomerization domains can be joined rigidly in different geometries. (c) A ribbon diagram of a fusion protein showing one method for joining two oligomerization domains (red and green) in a relatively rigid fashion. One of the natural oligomerization domains must end in an α-helical conformation, and the other must begin in an α-helical conformation. The two are then linked by a short stretch of amino acids (blue) that have a strong tendency to adopt an α-helical conformation. Thus, the two oligomerization domains are joined physically in a predictable orientation. (d) A designed fusion protein self assembles into a particular kind of nanostructure that depends on the geometry of the symmetry axes belonging to its component oligomerization domains (Table 1). A molecular layer arises from an arrangement like that in b (Left). (e) A cubic cage arises from an arrangement like the one in b (Right).