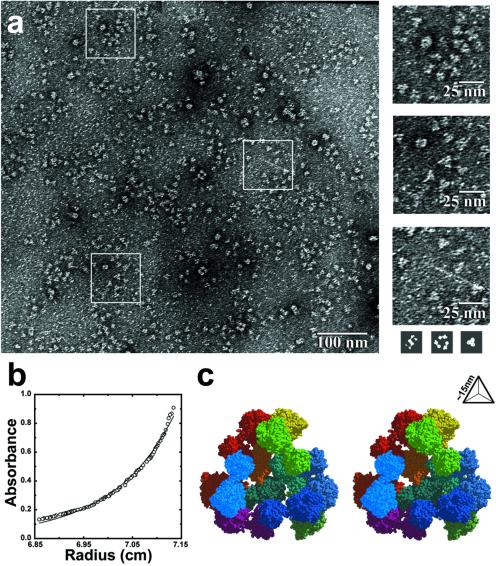
Figure 2.
Characterization of a designed tetrahedral protein cage. (a) Negatively stained electron micrographs show images of discrete particles. The images left in the heavy-atom stain are consistent with the sizes of the largest faces of the cage. For size comparison (shown to scale, Bottom Right), three simulated images were calculated from the atomic coordinates of the cage in three orientations where it would make the most extensive contacts with the surface of the electromagnetic support grid. As a rough approximation, it was assumed that the complex would leave a footprint in a layer of heavy-atom stain 15 Å thick. (b) Equilibrium sedimentation shows that the major component has a molecular mass of approximately 550 kDa, corresponding roughly to 11.3 subunits (close to the anticipated value of 12). A small degree of polymorphism is evident from the residual difference between the experimental and theoretical curves. (c) A stereo model of the tetrahedral protein cage as it was intended to assemble from 12 copies of the 49-kDa engineered fusion protein (shown in Fig. 1c). The view is through one of the four large openings in the cage. The particle radius is approximately 9 nm, and the edge length is approximately 15 nm. The separate protein subunits are colored differently.