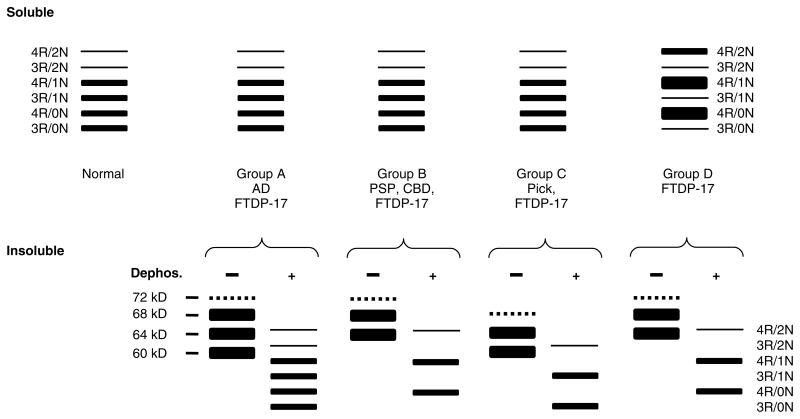
Figure 5.
Schematic representation of western blot banding patterns of soluble and insoluble tau from different tauopathies. The drawing depicts the typical banding pattern of soluble tau (top panels) and insoluble/filamentous tau (bottom panels) from the brains of patients with FTDP-17 as well as sporadic tauopathies following resolution with SDS-PAGE and immunoblotting with anti-tau antibodies. The FTDP-17 mutations show several different western blot banding patterns of soluble and insoluble tau protein that are depicted as groups A to D. The soluble fraction from the brains of unaffected (normal) individuals, sporadic tauopathies, and FTDP-17 with mutations that do not affect tau splicing (groups A, B, and C) shows expression of all six tau isoforms. Insoluble tau from the brains of patients with FTDP-17, group A (S320F, V337M, K369I, G389R, and R406W), resolves as three major proteins of 68, 64 and 60 kD; and a minor band of 72 kD similar to that observed in AD. When dephosphorylated, they resolve into six proteins that correspond to all six tau isoforms similar to the soluble fraction. In FTDP-17 group B (R5H, P301L, and G342V), two prominent 68- and 64-kD protein bands are detected (the 72 kD minor band is variably detected) that align with 4R tau following dephosphorylation similar to that observed in PSP and CBD, indicating the selective aggregation of 4R tau. In FTDP-17 group C (K257T) and Pick’s disease, the 64 and 60 kD insoluble tau protein isoforms predominate and align with 3R tau isoforms following dephosphorylation, indicating selective aggregation of 3R tau. In contrast, in FTDP-17 mutations that affect mRNA splicing (group D: N279K, L284L, N296N, N296H, S305S, S305N, and intron 10 mutations), there is expression of predominantly 4R tau throughout the entire brain, which is reflected in the insoluble tau aggregates