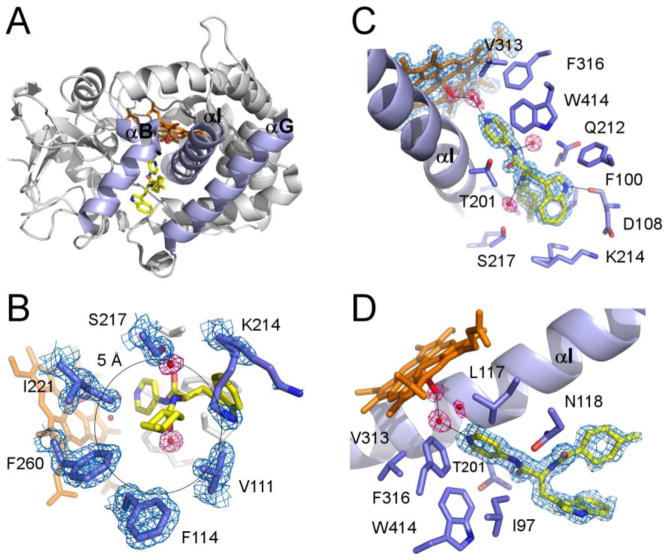
Fig. 4. Structure of the CYP125A1C429L-LP10 complex.
A. Overall ribbon structure of the CYP125A1C429L-LP10 complex (2XC3) with B, G and I helices highlighted in purple. LP10 (yellow), heme (orange) and the iron axial water ligand (red) are shown in stick mode. The pyridinyl ring of LP10 points toward heme. The indole and methylcyclohexyl moieties point toward the viewer between the B and G helices. B. A view of LP10 emphasizing the H-bonding interactions in the active site. Selected residues within 4 Å of LP10 are in purple. Alternate conformations of Lys214 interact with both indole and methylcyclohexyl moieties of LP10. C. Interactions of the methylcyclohexyl moiety within 5 Å of the methyl group. D. Catalytic chamber filled with three water molecules. In B, C and D, fragments of the 2Fo-Fc electron density map (calculated with LP10 coordinates omitted from the input file) are shown for heme, LP10, selected amino acid residues (blue mesh) and water molecules (pink mesh). Black lines highlight H-bonding interactions.