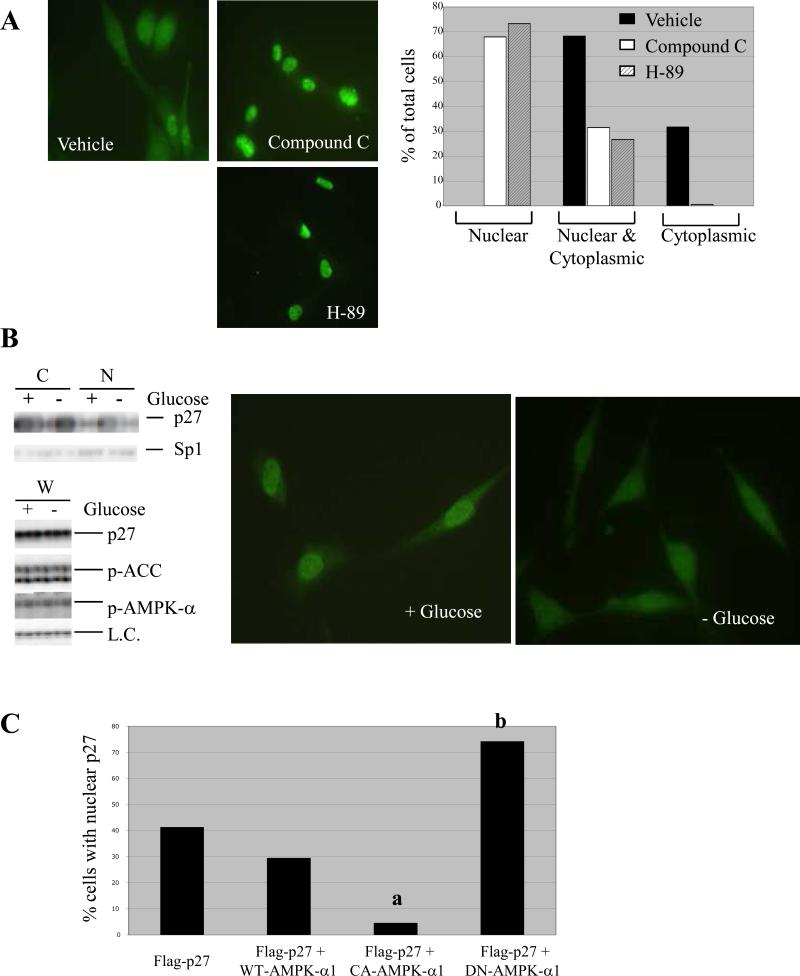
Figure 3.
AMPK signaling regulates subcellular localization of p27. (A) (left) Tuberin-null ELT-3 cells were treated for 5 hours with 100-nM H-89 or 20-μM Compound C and then visualized by immunocytochemistry using an anti-p27 antibody. Representative images (40X original magnification) are shown, and detection of p27 in the nucleus, cytoplasm, or both compartments was scored as a percentage of total cells analyzed. (B) Tuberin-expressing NIH3T3 cells were grown in media without serum in the presence or absence of glucose. Cells were then used to generate whole-cell (W), cytosolic (C) and nuclear (N) lysates. (top left) Cytosolic and nuclear lysates were immunoblotted using anti-p27 and anti-Sp1 antibodies. (bottom left) Whole-cell lysates were analyzed by immunoblotting using anti-p27, anti-p-AMPK-α (T172) and anti-p-ACC (S79) antibodies. Both p27 levels and levels of a non-specific band (L.C.) indicate equal loading. (right) NIH3T3 cells grown in the presence or absence of glucose were also analyzed by immunocytochemistry using an anti-p27 antibody. Representative images are shown (40x original magnification). (C) Tuberin-expressing NIH3T3 cells were transfected with Flag-p27-WT alone or in combination with HA-tagged WT-AMPK-α1, CA-AMPK-α1, or DN-AMPK-α1. Cells were then analyzed by immunocytochemistry using anti-Flag and anti-HA antibodies, and the percentage of cells expressing HA in which exogenous p27 was detected solely in the nucleus was determined. Statistical analysis (Fischer test) showed a significant decrease in the percentage of cells with nuclear Flag-p27-WT when CA-AMPK-α1 was overexpressed (a, p < 0.0001) and a significant increase in the percentage of cells with nuclear Flag-p27-WT when DN-AMPK-α1 was overexpressed (b, p < 0.0001).