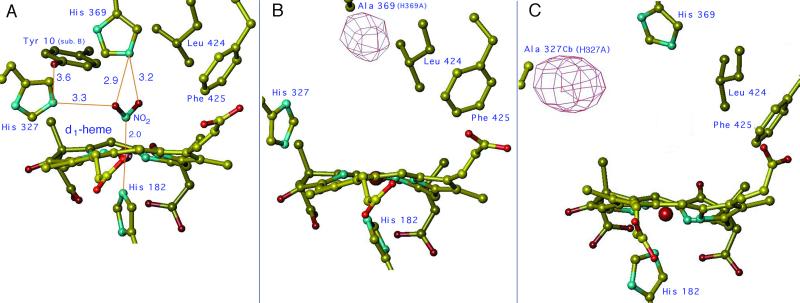
Figure 1.
The active site of cd1 NIR from P. aeruginosa (Pa-NIR). The model of the d1-heme pocket of the wt reduced enzyme complexed with nitrite is shown in A. The stereochemistry of NO2− was simulated starting from the coordinates of the NO adduct of reduced Pa-NiR (5). Among the key amino acid side chains shown here, notice that Tyr-10 comes from the other monomer (identified as sub. B), as a result of a domain swapping across the 2-fold axis of the homodimer (4). The 3D structure of the d1-heme pocket of the two mutants in the oxidized state is shown in the same orientation in B (for H369A) and C (for H327A). The Fo−Fc Sigma A negative electron density map is also represented at the place of the missing side chain; this map is contoured at −3σ for B and −4σ for C.