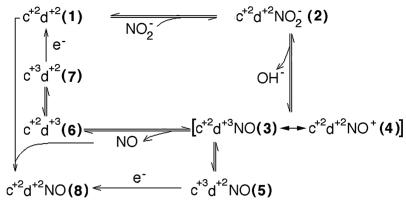
Figure 3.
A proposed scheme for the reaction mechanism of cd1 NIR with NO2−. When nitrite is mixed with the reduced enzyme (species 1), the formation of the Michaelis complex (species 2) is followed by very rapid formation of NO, involving bond breaking and loss of a hydroxide ion; yet this process is assumed to be reversible. The resulting mixed-valence species 3 or 4 (c+2d1+3 NO or c+2d1+2 NO+) may dissociate NO and be reduced to species 1 (via 6 and 7) to enter a new productive cycle; however, in vitro it seems to be progressively inhibited to a dead-end state with NO bound to the ferrous d1-heme (species 8). Species 1–5 equilibrate rapidly [see also George et al. (20)], accounting for a fraction of oxidized c-heme formed during the dead time of the stopped flow. The relative population of each species depends on experimental conditions, such as pH and concentration of substrate and reductant, and it may even differ in NIR from different species. The Michaelis complex, formed rapidly even at low nitrite concentrations (e.g., 100 μM), accounts for considerably less than 100% of the enzyme; therefore the bimolecular rate constant is fast, but the affinity is lower than previously suggested (24). Nevertheless, our kinetic data are consistent with the value of Km = 6 μM NO2−, which was independently determined (not shown). Species 6 builds up slowly (k = 2 s−1) and incompletely under our experimental conditions; the internal redox equilibrium between species 6 and 7 is assigned a rate constant of 0.3 s−1. Because it is well established that the electron accepting site is the c-heme, only species 5 and 7 can be reduced with the use of ascorbate, azurin, or cytochrome c551. In the scheme, there are two paths leading to c+2d1+2 NO, the dead-end species 8: either by reaction of the reduced enzyme with NO or by reduction of species 5 by external reductants. In P. pantotrotrophus NiR, species 5 forms completely and instantaneously at [NO2−] = 0.2–5.0 mM; thereafter it decays to species 4 at 38 s−1, as shown by George et al. (20); it is possible that a similar reequilibration also occurs in Pa-NIR, but if so, it is lost in the dead time.