Abstract
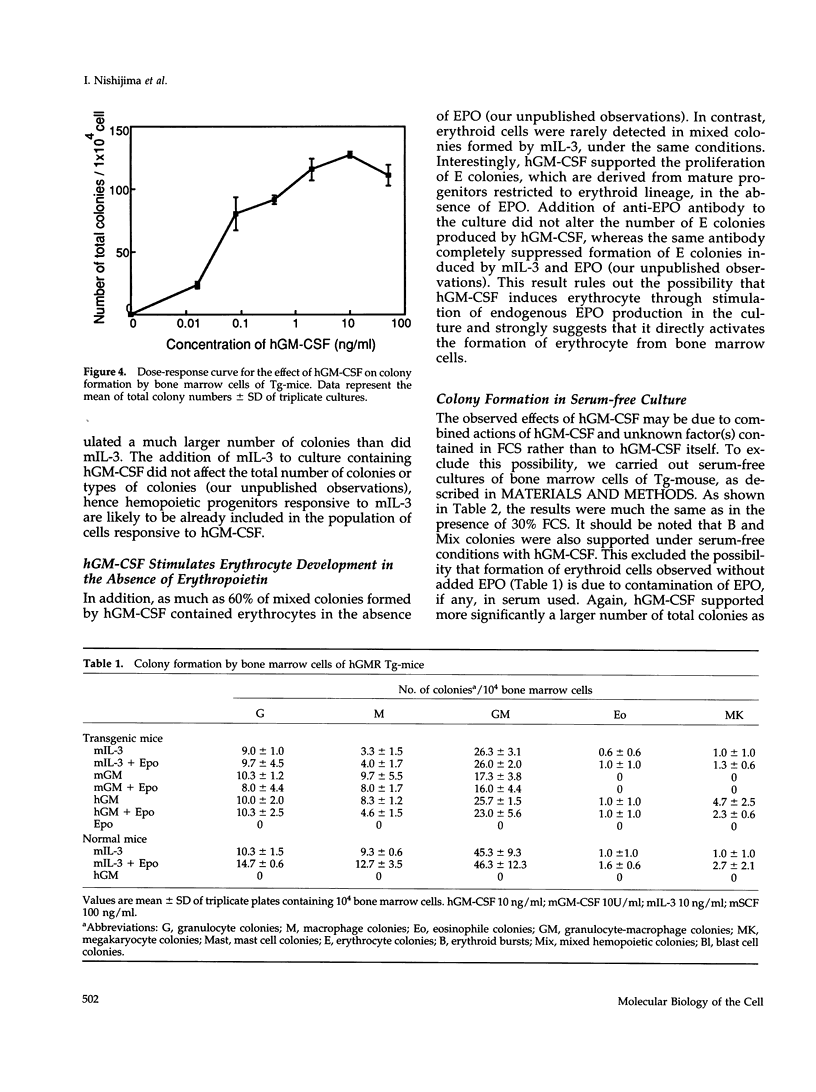
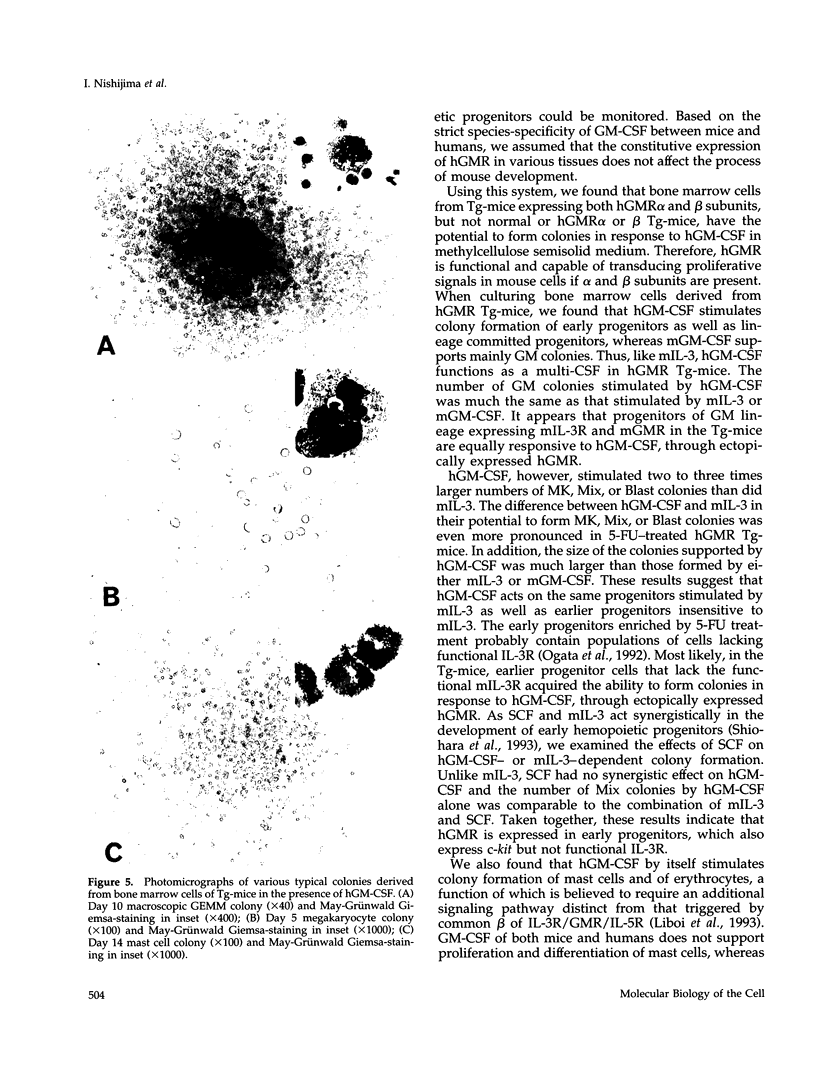
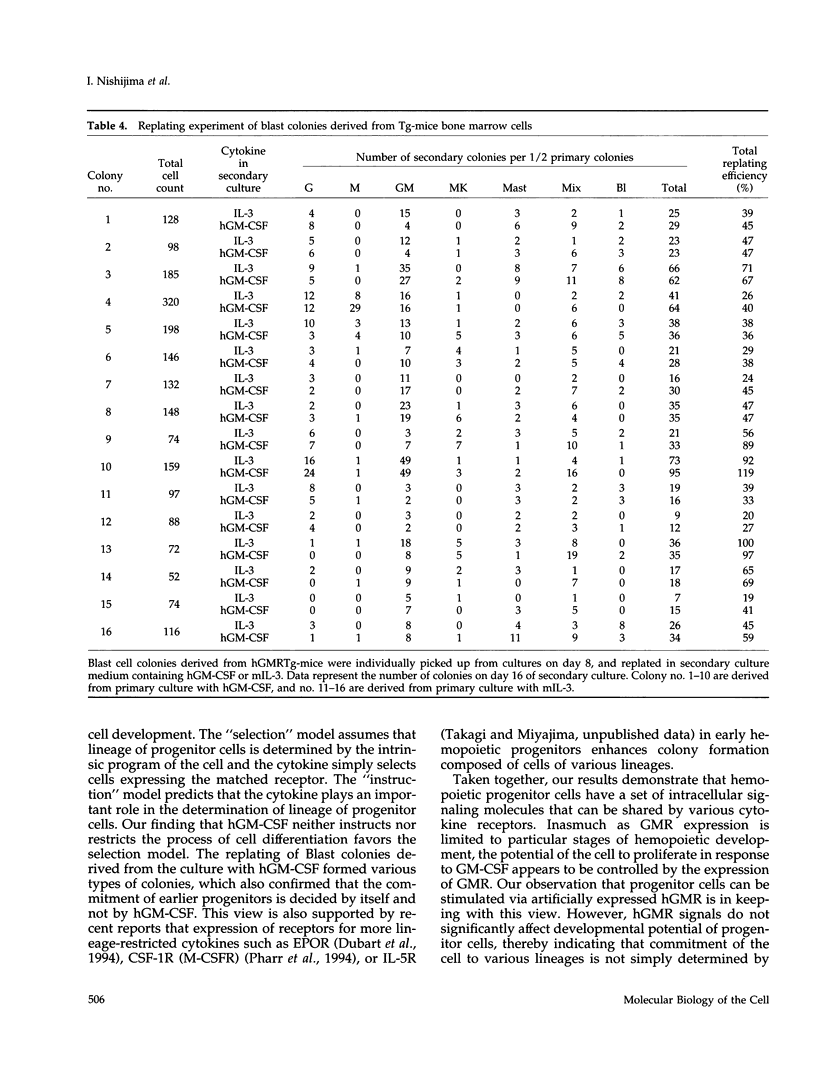
Granulocyte-macrophage colony-stimulating factor (GM-CSF) mainly stimulates proliferation and maturation of myeloid progenitor cells. Although the signal transduction pathways triggered by GM-CSF receptor (GMR) have been extensively characterized, the roles of GMR signals in differentiation have remained to be elucidated. To examine the relationship between receptor expression and differentiation of hemopoietic cells, we used transgenic mice (Tg-mice) that constitutively express human (h) GMR at almost all stages of hemopoietic cell development. Proliferation and differentiation of hemopoietic progenitors in bone marrow cells from these Tg-mice were analyzed by methylcellulose colony formation assay. High affinity GMR interacts with GM-CSF in a species-specific manner, therefore one can analyze the effects of hGMR signals on differentiation of mouse hemopoietic progenitors using hGM-CSF. Although mouse (m) GM-CSF yielded only GM colonies, hGM-CSF supported various types of colonies including GM, eosinophil, mast cell, erythrocyte, megakaryocyte, blast cell, and mixed hemopoietic colonies. Thus, the effects of hGM-CSF on colony formation more closely resembled mIL-3 than those of mGM-CSF. In addition, hGM-CSF generated a much larger number of blast cell colonies and mixed cell colonies than did mIL-3. hGM-CSF also generated erythrocyte colonies in the absence of erythropoietin. Therefore, GM-CSF apparently has the capacity to promote growth of cells of almost all hemopoietic cell lineages, if functional hGMR is present.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K. I., Lee F., Miyajima A., Miyatake S., Arai N., Yokota T. Cytokines: coordinators of immune and inflammatory responses. Annu Rev Biochem. 1990;59:783–836. doi: 10.1146/annurev.bi.59.070190.004031. [DOI] [PubMed] [Google Scholar]
- Broudy V. C., Lin N., Brice M., Nakamoto B., Papayannopoulou T. Erythropoietin receptor characteristics on primary human erythroid cells. Blood. 1991 Jun 15;77(12):2583–2590. [PubMed] [Google Scholar]
- Cannistra S. A., Groshek P., Garlick R., Miller J., Griffin J. D. Regulation of surface expression of the granulocyte/macrophage colony-stimulating factor receptor in normal human myeloid cells. Proc Natl Acad Sci U S A. 1990 Jan;87(1):93–97. doi: 10.1073/pnas.87.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dubart A., Feger F., Lacout C., Goncalves F., Vainchenker W., Dumenil D. Murine pluripotent hematopoietic progenitors constitutively expressing a normal erythropoietin receptor proliferate in response to erythropoietin without preferential erythroid cell differentiation. Mol Cell Biol. 1994 Jul;14(7):4834–4842. doi: 10.1128/mcb.14.7.4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasson J. C. Molecular physiology of granulocyte-macrophage colony-stimulating factor. Blood. 1991 Mar 15;77(6):1131–1145. [PubMed] [Google Scholar]
- Gearing D. P., King J. A., Gough N. M., Nicola N. A. Expression cloning of a receptor for human granulocyte-macrophage colony-stimulating factor. EMBO J. 1989 Dec 1;8(12):3667–3676. doi: 10.1002/j.1460-2075.1989.tb08541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman D. M., Itoh N., Kitamura T., Schreurs J., Yonehara S., Yahara I., Arai K., Miyajima A. Cloning and expression of a gene encoding an interleukin 3 receptor-like protein: identification of another member of the cytokine receptor gene family. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5459–5463. doi: 10.1073/pnas.87.14.5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida K., Kitamura T., Gorman D. M., Arai K., Yokota T., Miyajima A. Molecular cloning of a second subunit of the receptor for human granulocyte-macrophage colony-stimulating factor (GM-CSF): reconstitution of a high-affinity GM-CSF receptor. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9655–9659. doi: 10.1073/pnas.87.24.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Schreurs J., Gorman D. M., Maruyama K., Ishii A., Yahara I., Arai K., Miyajima A. Cloning of an interleukin-3 receptor gene: a member of a distinct receptor gene family. Science. 1990 Jan 19;247(4940):324–327. doi: 10.1126/science.2404337. [DOI] [PubMed] [Google Scholar]
- Jackson C. W. Cholinesterase as a possible marker for early cells of the megakaryocytic series. Blood. 1973 Sep;42(3):413–421. [PubMed] [Google Scholar]
- Kitamura T., Hayashida K., Sakamaki K., Yokota T., Arai K., Miyajima A. Reconstitution of functional receptors for human granulocyte/macrophage colony-stimulating factor (GM-CSF): evidence that the protein encoded by the AIC2B cDNA is a subunit of the murine GM-CSF receptor. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5082–5086. doi: 10.1073/pnas.88.12.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike K., Nakahata T., Takagi M., Kobayashi T., Ishiguro A., Tsuji K., Naganuma K., Okano A., Akiyama Y., Akabane T. Synergism of BSF-2/interleukin 6 and interleukin 3 on development of multipotential hemopoietic progenitors in serum-free culture. J Exp Med. 1988 Sep 1;168(3):879–890. doi: 10.1084/jem.168.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike K., Ogawa M., Ihle J. N., Miyake T., Shimizu T., Miyajima A., Yokota T., Arai K. Recombinant murine granulocyte-macrophage (GM) colony-stimulating factor supports formation of GM and multipotential blast cell colonies in culture: comparison with the effects of interleukin-3. J Cell Physiol. 1987 Jun;131(3):458–464. doi: 10.1002/jcp.1041310319. [DOI] [PubMed] [Google Scholar]
- Lang R. A., Metcalf D., Cuthbertson R. A., Lyons I., Stanley E., Kelso A., Kannourakis G., Williamson D. J., Klintworth G. K., Gonda T. J. Transgenic mice expressing a hemopoietic growth factor gene (GM-CSF) develop accumulations of macrophages, blindness, and a fatal syndrome of tissue damage. Cell. 1987 Nov 20;51(4):675–686. doi: 10.1016/0092-8674(87)90136-x. [DOI] [PubMed] [Google Scholar]
- Liboi E., Carroll M., D'Andrea A. D., Mathey-Prevot B. Erythropoietin receptor signals both proliferation and erythroid-specific differentiation. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11351–11355. doi: 10.1073/pnas.90.23.11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima A., Mui A. L., Ogorochi T., Sakamaki K. Receptors for granulocyte-macrophage colony-stimulating factor, interleukin-3, and interleukin-5. Blood. 1993 Oct 1;82(7):1960–1974. [PubMed] [Google Scholar]
- Miyajima A., Schreurs J., Otsu K., Kondo A., Arai K., Maeda S. Use of the silkworm, Bombyx mori, and an insect baculovirus vector for high-level expression and secretion of biologically active mouse interleukin-3. Gene. 1987;58(2-3):273–281. doi: 10.1016/0378-1119(87)90382-9. [DOI] [PubMed] [Google Scholar]
- Nakahata T., Kobayashi T., Ishiguro A., Tsuji K., Naganuma K., Ando O., Yagi Y., Tadokoro K., Akabane T. Extensive proliferation of mature connective-tissue type mast cells in vitro. Nature. 1986 Nov 6;324(6092):65–67. doi: 10.1038/324065a0. [DOI] [PubMed] [Google Scholar]
- Nakahata T., Ogawa M. Clonal origin of murine hemopoietic colonies with apparent restriction to granuclocyte-macrophage-megakaryocyte (GMM) differentiation. J Cell Physiol. 1982 Jun;111(3):239–246. doi: 10.1002/jcp.1041110304. [DOI] [PubMed] [Google Scholar]
- Nakahata T., Ogawa M. Identification in culture of a class of hemopoietic colony-forming units with extensive capability to self-renew and generate multipotential hemopoietic colonies. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3843–3847. doi: 10.1073/pnas.79.12.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata T., Spicer S. S., Cantey J. R., Ogawa M. Clonal assay of mouse mast cell colonies in methylcellulose culture. Blood. 1982 Aug;60(2):352–361. [PubMed] [Google Scholar]
- Nakeff A., Daniels-McQueen S. In vitro colony assay for a new class of megakaryocyte precursor: colony-forming unit megakaryocyte (CFU-M). Proc Soc Exp Biol Med. 1976 Mar;151(3):587–590. doi: 10.3181/00379727-151-39265. [DOI] [PubMed] [Google Scholar]
- Ogata H., Taniguchi S., Inaba M., Sugawara M., Ohta Y., Inaba K., Mori K. J., Ikehara S. Separation of hematopoietic stem cells into two populations and their characterization. Blood. 1992 Jul 1;80(1):91–95. [PubMed] [Google Scholar]
- Ogawa M. Differentiation and proliferation of hematopoietic stem cells. Blood. 1993 Jun 1;81(11):2844–2853. [PubMed] [Google Scholar]
- Pharr P. N., Ogawa M., Hofbauer A., Longmore G. D. Expression of an activated erythropoietin or a colony-stimulating factor 1 receptor by pluripotent progenitors enhances colony formation but does not induce differentiation. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7482–7486. doi: 10.1073/pnas.91.16.7482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPICER S. S. A correlative study of the histochemical properties of rodent acid mucopolysaccharides. J Histochem Cytochem. 1960 Jan;8:18–35. doi: 10.1177/8.1.18. [DOI] [PubMed] [Google Scholar]
- Saito H., Hatake K., Dvorak A. M., Leiferman K. M., Donnenberg A. D., Arai N., Ishizaka K., Ishizaka T. Selective differentiation and proliferation of hematopoietic cells induced by recombinant human interleukins. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2288–2292. doi: 10.1073/pnas.85.7.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiohara M., Koike K., Nakahata T. Synergism of interferon-gamma and stem cell factor on the development of murine hematopoietic progenitors in serum-free culture. Blood. 1993 Mar 15;81(6):1435–1441. [PubMed] [Google Scholar]
- Smith L. G., Weissman I. L., Heimfeld S. Clonal analysis of hematopoietic stem-cell differentiation in vivo. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2788–2792. doi: 10.1073/pnas.88.7.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T., Suda J., Ogawa M. Proliferative kinetics and differentiation of murine blast cell colonies in culture: evidence for variable G0 periods and constant doubling rates of early pluripotent hemopoietic progenitors. J Cell Physiol. 1983 Dec;117(3):308–318. doi: 10.1002/jcp.1041170305. [DOI] [PubMed] [Google Scholar]
- Suda T., Suda J., Ogawa M. Single-cell origin of mouse hemopoietic colonies expressing multiple lineages in variable combinations. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6689–6693. doi: 10.1073/pnas.80.21.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suematsu S., Matsusaka T., Matsuda T., Ohno S., Miyazaki J., Yamamura K., Hirano T., Kishimoto T. Generation of plasmacytomas with the chromosomal translocation t(12;15) in interleukin 6 transgenic mice. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):232–235. doi: 10.1073/pnas.89.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka R., Koike K., Imai T., Shiohara M., Kubo T., Amano Y., Komiyama A., Nakahata T. Stem cell factor enhances proliferation, but not maturation, of murine megakaryocytic progenitors in serum-free culture. Blood. 1992 Oct 1;80(7):1743–1749. [PubMed] [Google Scholar]
- Walker F., Burgess A. W. Specific binding of radioiodinated granulocyte-macrophage colony-stimulating factor to hemopoietic cells. EMBO J. 1985 Apr;4(4):933–939. doi: 10.1002/j.1460-2075.1985.tb03721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker F., Nicola N. A., Metcalf D., Burgess A. W. Hierarchical down-modulation of hemopoietic growth factor receptors. Cell. 1985 Nov;43(1):269–276. doi: 10.1016/0092-8674(85)90032-7. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Mui A. L., Muto A., Chen J. X., Hayashida K., Yokota T., Miyajima A., Arai K. Reconstituted human granulocyte-macrophage colony-stimulating factor receptor transduces growth-promoting signals in mouse NIH 3T3 cells: comparison with signalling in BA/F3 pro-B cells. Mol Cell Biol. 1993 Mar;13(3):1440–1448. doi: 10.1128/mcb.13.3.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Kitamura T., Hayashida K., Miyajima A. Monoclonal antibody against the common beta subunit (beta c) of the human interleukin-3 (IL-3), IL-5, and granulocyte-macrophage colony-stimulating factor receptors shows upregulation of beta c by IL-1 and tumor necrosis factor-alpha. Blood. 1992 Nov 1;80(9):2215–2220. [PubMed] [Google Scholar]