FIGURE 8. Three-dimensional structure of the PCI domain in human eIF3k.
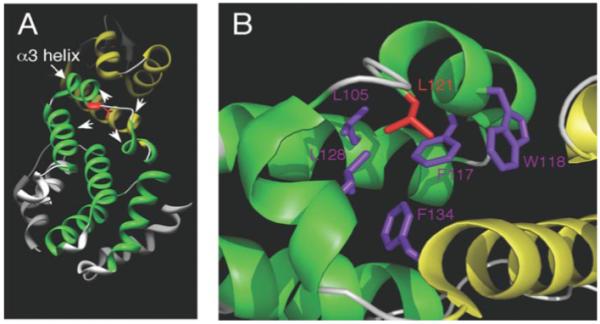
A, the N-terminal TPR/HAM subdomain is colored green, whereas the C-terminal WH subdomain is colored yellow. The described leucine residue corresponds to Leu121 (red) in eIF3k and is located at the end of the α3 helix in the N-terminal TPR/HAM subdomain. Three helices from the TPR-like subdomain and one helix from the WH subdomain (arrowheads) contain hydrophobic amino acid residues that may interact with Leu121 (see B). B, within a 5-Å radius from this leucine, at least 5 hydrophobic amino acids colored in purple (Leu105, Phe117, Trp118, Leu128, and Phe134) can be readily identified that may interact with this leucine. We note that although most of these neighboring hydrophobic residues reside in the TPR/HAM domain, Phe134 is in the WH domain. These images were created by Chimera (32) and PyMOL software.
