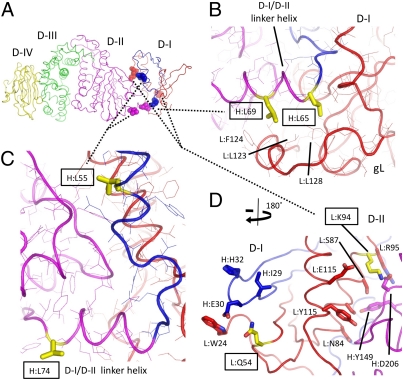
Fig. 3.
D-I residues implicated in membrane fusion activity of gH/gL. (A) Residues implicated in gH/gL function are shown as semitransparent CPK spheres colored by chain and domain as in Fig 1. The backbone trace is represented as coils, and the residues are labeled as described in Fig. 1. (B) Mutations of EBV gH L65A and L69A, referred to as H:L65 and H:L69, respectively, reduce gH/gL membrane fusion activity with both B cells and epithelial cells. H:L65 and H:L69 are shown in yellow sticks, with surrounding residues shown as lines colored by the gH/gL chain as in A and labeled. Both residues form hydrophobic contacts with gL at the edge of the D-I hydrophobic core. (C) gH mutants L55A and L74A enhance viral fusion activity. H:L55 is located at the D-I/D-II interface, whereas H:L75 is exposed on the surface of the D-I/D-II linker helix at the beginning of D-II. Residues are colored as in B. (D) EBV gL Q54 and K94 (yellow sticks and boxes) control the specificity of gB activation in membrane fusion. Mutation to Rhesus lymphocryptovirus (LCV) gL (82% identity with EBV gL) residues K54 and Q94 reduces membrane fusion with EBV gB. L:Q54 is exposed at the surface of D-I in the loop between the first and second β-strands (Lβ-1 and Lβ-2). Adjacent residues (L:W24, H:I29, H:E30, and H:H32) are also surface-exposed and form a prominent ridge adjacent to Q54. K94 is located at the D-I/D-II interface in an area rich in polar and charged residues (as described in the text). L:Q54 and L:K94 are show as yellow sticks, with identified neighboring residues shown as sticks colored by their associated chain and domain.