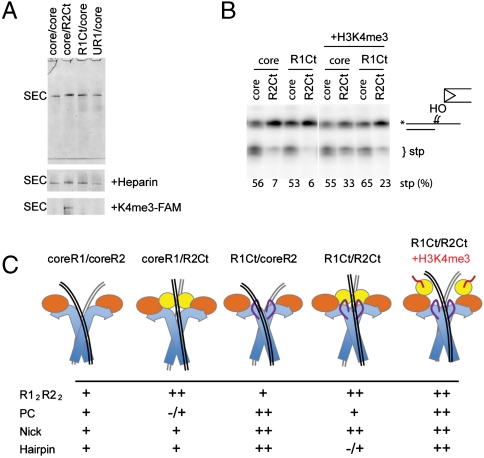
Fig. 6.
Properties of postcleavage complex with noncore domains. (A) Coomassie stained native gel of purified SEC produced by an on-column reaction, without and with heparin challenge (second panel). The third panel shows the fluorescent H3K4me3 peptide colocalized with SEC that contained PHD. (B) Effect of noncore domains on strand-transfer activity of postcleavage complexes. The target DNA is a 3′ overhang substrate that is cleaved when SEC transposes the signal end at the ds/ss junction. On addition of H3K4me3 the inhibition by RAG2Ct was alleviated. (C) Schematic model for inhibition mechanism supported by data: (i) The incomplete catalytic domain of core R1/coreR2 allows flexibility of substrates. (ii) The PHD domain of RAG2 alters coding flank contacts and restricts the position of the transesterification site. (iii) The R1Ct increases affinity with the signal sequence. (iv) Together the RAG1 and RAG2 C-terminal regions produce a tighter complex with 12/23RSS that restricts the distortions required for hairpinning. (v). When H3K4me3 is bound, the PHD is released from its inhibitory position allowing hairpinning to proceed. The arrangement of domains and DNAs was elucidated previously by electron microscopy (18).