Abstract
Increased farm salmon production has heightened concerns about the association between disease on farm and wild fish. The controversy is particularly evident in the Broughton Archipelago of Western Canada, where a high prevalence of sea lice (ectoparasitic copepods) was first reported on juvenile wild pink salmon (Oncorhynchus gorbuscha) in 2001. Exposure to sea lice from farmed Atlantic salmon (Salmo salar) was thought to be the cause of the 97% population decline before these fish returned to spawn in 2002, although no diagnostic investigation was done to rule out other causes of mortality. To address the concern that sea lice from fish farms would cause population extinction of wild salmon, we analyzed 10–20 y of fish farm data and 60 y of pink salmon data. We show that the number of pink salmon returning to spawn in the fall predicts the number of female sea lice on farm fish the next spring, which, in turn, accounts for 98% of the annual variability in the prevalence of sea lice on outmigrating wild juvenile salmon. However, productivity of wild salmon is not negatively associated with either farm lice numbers or farm fish production, and all published field and laboratory data support the conclusion that something other than sea lice caused the population decline in 2002. We conclude that separating farm salmon from wild salmon—proposed through coordinated fallowing or closed containment—will not increase wild salmon productivity and that medical analysis can improve our understanding of complex issues related to aquaculture sustainability.
Because salmon aquaculture production has rapidly increased over the past three decades, the potential for environmental impacts of salmon farms has generated heightened scientific and public interest (1, 2). One concern about salmon farms is that they are the source of ectoparasitic sea lice infestations that might reduce the marine survival of wild salmon (3, 4). In the Broughton Archipelago region of Western Canada (Fig. S1), farming of Atlantic salmon (Salmo salar) began in the late 1980s, and annual farm salmon production increased steadily to 17 Gg by 1999 (Fig. S2). Pink salmon (Oncorhynchus gorbuscha) is the most abundant wild salmon species in the Broughton Archipelago; they enter the marine environment at a very small size (0.2 g), and they return to natal streams to spawn 2 y after their parents (5). Because age at maturity never varies, they have distinct even- and odd-year populations (Fig. S2 and SI Text). Record high numbers of pink salmon returned to spawn in rivers of the Broughton Archipelago in 2000 and 2001 (Dataset S1), but these returns were followed by population decline of 97% in 2002 and 88% in 2003 (Figs. S2 and S3 and SI Text). When juvenile pink salmon in the Broughton Archipelago were first examined for sea lice in June 2001, more than 90% were infested—leading to the hypothesis that sea lice from fish farms were the cause of population collapse in 2002 (4).
Adult pink salmon are a natural host for the sea louse species Lepeophtheirus salmonis (6), and in Western Canada, L. salmonis is generally common on all mature salmon returning to the coast (7, 8). In contrast, L. salmonis is rare on juvenile pink salmon in areas with no fish farms (9). L. salmonis occurs in the Atlantic and Pacific oceans, but the Pacific form is clinically less pathogenic than the Atlantic form (10), and the two forms have significant genetic differences (11, 12). One other sea louse species, Caligus clemensi, occurs on pink salmon, but it is more common on other fish hosts (13). Unlike L. salmonis, C. clemensi is sometimes common on juvenile pink salmon away from fish farms (13).
Several studies have attempted to explain the impact of sea lice and salmon farming on pink salmon population decline, but these studies have been limited by lack of access to fish farm data (14–17). In one series of studies, juvenile pink salmon of unknown history were captured from the wild (2004–2007), separated by lice infestation status into field-based enclosures, and held for several weeks to assess differences in mortality (14, 18, 19). Results from these studies were used to support the conclusion that “recurrent louse infestations of wild juvenile pink salmon (Oncorhynchus gorbuscha), all associated with salmon farms, have depressed wild pink salmon populations and placed them on a trajectory toward rapid local extinction” (3). However, the field mortality studies were not able to differentiate whether sea lice were the cause of mortality or whether sea lice had preferentially attached to fish that were destined to die from some other cause. To overcome this deficiency, other research exposed juvenile pink salmon of known history to Pacific forms of L. salmonis under controlled laboratory conditions (20); results were used to estimate that sea lice killed no more than 4.5% of juvenile pink salmon in any given year from 2005 to 2008 (21). Conclusions from these studies remain controversial, in part because they depend on experimental results from confined wild fish. Pink salmon sometimes adapt poorly to confinement (22) and change their behavior when exposed to sea lice (23); therefore, experimental results might overestimate or underestimate mortality among lice-infested fish in the wild. To overcome limitations inherent in using experimental studies to estimate population outcomes, we use a combination of approaches common in medical science and mathematical modeling to analyze actual farm data in relation to wild salmon information.
The primary objective of our study was to assess interrelationships between wild pink salmon, sea lice, and farmed Atlantic salmon in the Broughton Archipelago. Most importantly, we wanted to determine whether farm-source sea lice negatively impacted wild salmon population productivity (measured by the number of returning fish per spawner). Our approach was to (i) identify trends in monthly and annual sea lice numbers on farm fish, (ii) determine the relationship of these trends to medical treatments prescribed to kill sea lice, (iii) identify the progressive relationship between run-specific adult pink salmon returns in the fall, farm salmon lice numbers the next April, and sea lice prevalence on juvenile pink salmon 1 mo later (in May), and (iv) assess whether farm fish production or sea lice numbers could be used to improve predictions of pink salmon returns by using a generalization of the traditional Ricker spawner–recruit relationship. Finally, we compared our data with all available experimental data to determine the potential benefit of policy options that have been proposed to decrease the impact of farm salmon on wild salmon abundance.
Results and Discussion
Farm Fish Inventories and Sea Lice Numbers.
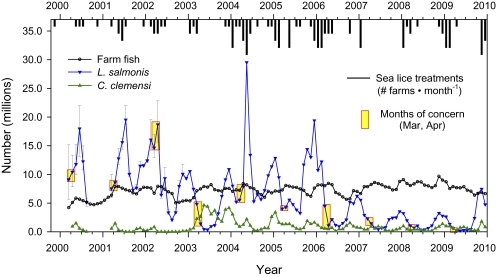
The total number of sea lice on farm fish was considerably more variable than the total number of farm fish in the Broughton Archipelago (data from all farms combined) from 2000 to 2009 (Fig. 1). The largest monthly estimate of total adult female L. salmonis numbers on all Broughton farms over the past decade was 180 times greater than the smallest estimate (29.5 million in May 2004 vs. 164,500 in April 2009), but the largest total farm fish inventory was only 2.3 times greater than the smallest inventory (9.7 million fish in October 2008 vs. 4.2 million fish in March 2000). This suggests that variation in sea lice numbers on farm fish is a result of something other than numbers of farm fish. L. salmonis was the dominant louse species on farm fish during all months except for April to August 2003 (Fig. 1), when estimates for adult female C. clemensi were more than 1 million lice greater than for L. salmonis.
Fig. 1.
Monthly total number of farm fish, farm-source adult female sea lice, and sea lice treatments (n = 0–5 per mo; axis from top of figure not shown) in the Broughton Archipelago. L. salmonis numbers during the time of greatest concern (March and April) are highlighted in yellow. Error bars for L. salmonis estimates before 2003 denote the range of uncertainty during this period, when lice were not counted on every farm.
Sea lice numbers on farm fish in the Broughton Archipelago changed over time (Fig. 1), but the pattern within farms was different from the pattern when all area farm data were combined. Farm salmon enter the marine environment free of sea lice (24), but sea lice in the environment start to infest the fish within a few months of stocking (10). Within each farm, the monthly total number of adult female L. salmonis either remained stable—when freshwater runoff decreased the salinity (and louse replication) at many farms (10)—or increased, probably from (i) ongoing environmental exposure to new lice and (ii) maturation of young lice already on the fish; louse numbers decreased only when fish were treated or removed from the farm (Dataset S1). In comparison, among all farms combined, the total number of adult female L. salmonis changed in an annual cycle that began in late summer (September). During all years, L. salmonis numbers were relatively low in late summer (Fig. 1). Louse numbers steadily increased (i) in the fall, when adult wild salmon (infested with lice) (7, 8) returned to the area to spawn, and (ii) in the winter, when increased salinity favored louse reproduction (24).
Treatments Decrease Farm Fish Sea Lice Numbers.
Sea lice treatments occurred in a distinct annual pattern, and treatments decreased sea lice numbers within about 2 mo. We designated September as the beginning of the annual cycle, before the fall increase in sea lice numbers. Farm fish were never treated for sea lice in September, but treatment frequency increased in the fall and winter and then decreased during the summer (Fig. S4). The greatest total number of adult female sea lice on all farms in the Broughton Archipelago occurred in May 2004, when the total adult female L. salmonis abundance on just two farms (18.7 million) was greater than the total for all 17 active farms in the Broughton Archipelago 1 mo before (in April; 7.9 million). The abrupt increase at these farms is evidence for an uncommon source—possibly wild fish—that year in the northern part of the Broughton Archipelago. Emamectin benzoate (Slice) treatments at these two farms in late May rapidly decreased their female L. salmonis counts to 5.9 million in June and 0.75 million by July (Dataset S1). From 2000 to 2009, emamectin benzoate treatments on other farms were consistently effective (Dataset S1).
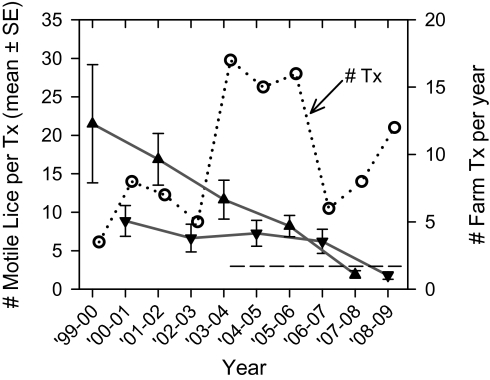
Over the past decade, sea lice treatments tended to occur progressively earlier within the annual cycle, and this change was associated with earlier peaks within the annual cycle for Broughton-wide total L. salmonis numbers. The average time from the beginning of the cycle (September) to the month of treatment application decreased from 7.1 mo (April during 1999–2001) to 5.7 mo (late February during 2002–2005) and finally, to 3.8 mo (late December during 2005–2009) (Dataset S1). Before 2003, veterinarians prescribed treatments solely to decrease the effects of lice on farm salmon; treatments were applied later in the annual cycle, because it usually took several months for lice infestations to reach a level that adversely affected farm salmon. Since 2004, government regulations required treatment whenever infestation levels exceeded three motile L. salmonis per fish during juvenile salmon outmigration (March through June). By the September 2008 to August 2009 cycle, treatments were applied to most farms, regardless of lice levels: among the 12 farms treated during that year, only two had more than three motile L. salmonis per fish at the time of treatments (Fig. 2 and Dataset S1). As treatment patterns changed, the month of peak L. salmonis numbers during the annual cycle moved backward from June/July (2000 and 2001) to April (2002) and finally, to November (2008 and 2009) (Fig. 1). It is unlikely that changes in environmental sources of sea lice (e.g., other wild fish species) or changes in environmental conditions (e.g., salinity or temperature) would be sufficient to cause this 7-mo shift (from June back to November) in peak L. salmonis numbers.
Fig. 2.
Mean number of motile L. salmonis sea lice per fish on farms that were treated after odd-year pink salmon runs (triangles) and even-year pink salmon runs (inverted triangles) and the total number of sea lice treatments (Tx; open circles) per year (September 1 to August 31). The treatment threshold (number of motile L. salmonis per fish; dashed line) for March through June was established by the British Columbia provincial government in 2004.
Adult Pink Salmon Are a Potential Source for Fish Farm Sea Lice.
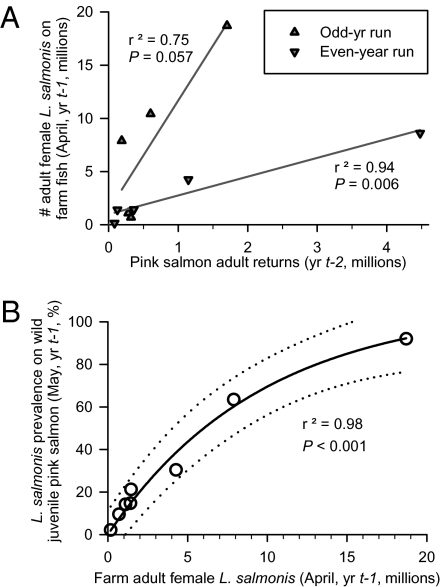
Although sea lice from environmental sources probably have little effect on the timing of the annual peak in sea lice abundance on farm salmon, the magnitude of the annual cycle is associated with adult pink salmon returns. As evidence, the number of adult pink salmon returning to the Broughton Archipelago in the fall (1999–2008) is correlated with the numbers of sea lice infesting farm salmon the next spring, and the odd-year run is associated with more sea lice on farm fish than the even-year run (Fig. 3A). In support, during the early part of the past decade, odd-year runs tended to be associated with more emamectin benzoate treatments at higher L. salmonis infestation levels than even-year runs (Fig. 2). The relationship between adult returns and next-spring farm sea lice numbers is statistically significant or almost significant within each run (Fig. 3A), and slopes of the lines for each run are significantly different (P = 0.02) (Dataset S1). When all Broughton returns—even- and odd-year runs combined—are compared against April farm lice numbers using a simple regression, the relationship is not significant (P = 0.18, r2 = 0.21).
Fig. 3.
(A) Adult pink salmon returns in the fall (1999–2008) and total numbers of adult female L. salmonis sea lice on farm fish the next April (2000–2009). (B) Total number of adult female L. salmonis on farm fish in April and prevalence of L. salmonis on wild juvenile pink salmon in May (2002–2009; ± 95% prediction interval).
Run-specific differences suggest that pink salmon contribute more sea lice to farm fish during the odd-year run. In support, pink salmon abundance and sea lice levels were greater in off-shore waters in the North Pacific during the odd years (1991–1997 studied) (25). In the Broughton Archipelago, the mean number of gravid (egg-bearing) female L. salmonis per adult pink salmon was greater in 2003 (9.9) than in 2004 (7.1), the only years from which these data are available (7, 8). Also, the nearby Fraser River (Fig. S1) has only odd-year runs of pink salmon, and from 1999 to 2009, runs there varied from 3.6 to 26 million fish. These large populations of pink salmon might contribute sea lice to farm fish in the Broughton Archipelago. When Fraser River and Broughton Archipelago pink salmon returns are combined in a multiple regression analysis with even- and odd-year runs combined, the relationship between pink salmon returns and Broughton farm L. salmonis numbers the next April is almost significant (P = 0.057), and the regression explains more of the variability (r2 = 0.56) (Dataset S1).
Variation in the farm sea lice numbers in the fall is not consistently related to variation in pink salmon returns; however, above-median pink salmon returns in 2009 (Fig. S2) were associated with greater farm lice numbers in November 2009 than in any month since February 2006 (Fig. 1). Progressively more aggressive and coordinated treatments of sea lice on farm fish have truncated the relationship between pink salmon returns in the fall and farm fish sea lice numbers in the spring. For example, as escapement of the odd-year run in the Broughton Archipelago increased slightly over the years 2003, 2005, and 2007 (Fig. S2), sea lice numbers on farm fish the next April decreased over the years 2004, 2006, and 2008 (Fig. 1).
Relationship of Sea Lice on Farm Fish and Juvenile Pink Salmon.
L. salmonis prevalence on outmigrating juvenile pink salmon in May shows a strong curvilinear relationship with adult female L. salmonis abundance on farm fish in April (r2 = 0.981, a = 107.7, b = 1.04 × 10−7) (Fig. 3B). This relationship remained stable as progressively earlier farm lice treatments truncated the timing of the annual peak in farm fish L. salmonis abundance from the spring (in 2002) back to late fall (by 2008/2009). The L. salmonis prevalence on juvenile pink salmon was not determined in May 2001, but the reported prevalence of 96% in June and July 2001 (4) is within the 95% prediction interval based on our estimate of 15.6 million female L. salmonis on farm fish in June 2001 (Figs. 1 and 3B). Collectively, these findings support the hypothesis that farm fish are the main source of L. salmonis infesting juvenile pink salmon, and they are consistent with indirect evidence from several other studies that did not have access to farm data (14, 16, 26, 27).
Because farm-source sea lice accounted for 98% of the variability in wild salmon sea lice prevalence from 2002 to 2009 and sea lice were sometimes common on farmed Atlantic salmon during the 1990s, farm-source sea lice probably infested juvenile pink salmon many years before they were first examined for sea lice in 2001 (1). As evidence, we show that sea lice were abundant on farm fish in 2000 (Fig. 1). Before 2000, farm fish sea lice were usually not quantified, but infestations were common enough that sea lice treatment options were investigated in the early 1990s (28), and publicly available records confirm that those treatments were used as early as 1996 (figure 25 at http://www.agf.gov.bc.ca/ahc/fish_health/Fish_Health_Report_2003-2005.pdf). No evidence exists to indicate that sea lice are not moving through net cages in both directions between farm and wild fish (16), and net cages have not changed over the past two decades.
Relationship of Farm Fish Production and Farm Sea Lice to Pink Salmon Productivity.
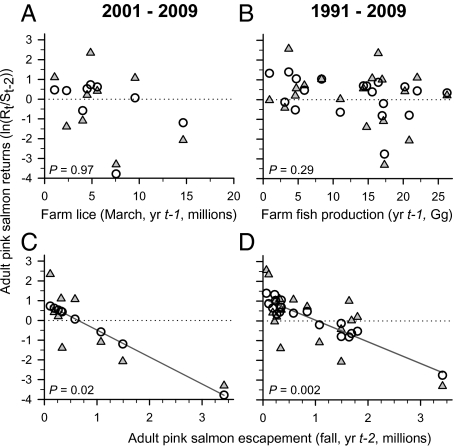
Despite the strong correlation between sea lice infestation of farm salmon and wild salmon, pink salmon productivity is not consistently associated with either farm fish adult female L. salmonis numbers (March) or farm fish production (Gg·y−1) during the year of juvenile outmigration (Fig. 4). Instead, pink salmon returns per spawner (year t) are significantly associated with escapement of the parent generation (t − 2) for returns of the odd- and even-year runs combined in (i) 2001–2009 (the period corresponding to sea lice counts; P = 0.02) and (ii) 1991–2009 (the period corresponding to fish farm production data; P = 0.002) (Fig. 4). When odd- and even-year populations are analyzed separately, the relationship of pink salmon returns to parental escapement is significant during odd years from 1991 to 2009 (P = 0.02) and almost significant during even years from 1992 to 2008 (P = 0.08). For all combinations of years and runs (even or odd year), pink salmon adult returns (year t) were never significantly associated with year t − 1 farm fish production (P ≥ 0.27). The only significant relationship with farm adult female L. salmonis numbers was a positive correlation with the even-year run from 2002 to 2008 (P = 0.04) (Dataset S1). Positive contributions of sea lice to pink salmon productivity have not been investigated, but sea lice might be a food source. As evidence, juvenile pink salmon held in captivity feed on sea lice attached to cohorts (19).
Fig. 4.
Observed values (closed triangles), predicted values (open circles), and P values for the two multiple linear regressions of pink salmon production [generational survival; ln (Rt − 2/St)]. (i) For 2001–2009 data, the relationship of generational survival to farm-source adult female L. salmonis sea lice was not significant (A), but the relationship of generational survival to parental escapement (St − 2) was significant (C). (ii) For 1991–2009 data, the relationship of generational survival to farm fish production was not significant (B), but the relationship of generational survival to parental escapement was significant (D). Dotted lines denote where returns equal parental escapement. For emphasis, solid trend lines are shown for significant relationships (C and D).
Medical Analysis of Potential Causes of Pink Salmon Mortality.
The data from Broughton Archipelago pink salmon populations and sea lice experiments best fit the conclusion that the majority of pink salmon deaths are caused by something other than sea lice, and our farm data supports the conclusion that farm lice did not significantly decrease pink salmon productivity over the past decade. Historically, mortality from fry to adult for central British Columbia stocks is about 95% (29), and most of this mortality occurs during the first 6 wk in the ocean (30). When lice-infested juvenile pink salmon of unknown health status were captured from the marine environment and held for several days in field-based enclosures, mortality was consistently higher among fish that began the experiment infested with sea lice; however, the parasites were shed from the majority (19) or all (14, 18) of the fish that eventually died. Koch's fourth postulate states that, to confirm a parasite as the cause of disease, presence of the parasite must be confirmed on the diseased host (31). Because most or all of the initially infested fish shed their lice before they died, the cause of death remains unknown, and the evidence points to something other than sea lice killing most of the fish. These experiments did not include diagnostic methods (e.g., bacteriology, virology, or histopathology) to identify other causes of death. In some cases, Koch's postulates need not be fulfilled to establish disease causation (e.g., with bacteria and viruses that cannot be cultured), but sea lice are routinely isolated for exposing lice-free fish under controlled laboratory conditions (23, 32).
To better determine the role of sea lice in pink salmon mortality (e.g., do they contribute to or cause mortality?), controlled laboratory experiments exposed lice-free juvenile pink salmon of known history to Pacific-source L. salmonis. Infested fish had increased jumping activity and a slight preference for fresh water (23), and infested juvenile pink salmon shed most lice within about 3 wk (32); mortality was limited to heavily infested fish weighing <0.7 g (20, 32). Therefore, because of their rapid growth rate, pink salmon are susceptible to lethal sea lice infestations only during their first 1 mo at sea (21). Based on extrapolations from controlled laboratory studies, infestation levels associated with our estimate of 9.5 million farm-source female L. salmonis in February 2005 (Fig. 1) might have killed 8% of the juvenile salmon outmigrating in March 2005 (21). However, generational escapement for these fish returning as adults in 2006 was similar for the Broughton and reference populations (Fig. S3), suggesting that, if louse-induced mortality occurred, it was compensatory (i.e., death caused by sea lice exposure replaced death caused by other causes, resulting in no net change in generational survival).
Assessment of the Potential for Management Options to Improve Pink Salmon Abundance.
Two management options are being considered for decreasing the impact of farm salmon on wild salmon (1, 2, 19): (i) coordinated fallowing of juvenile salmon migration corridors and (ii) removal of all farms from direct contact with the marine environment (e.g., through transition from open net cages to closed containment aquaculture). After severe pink salmon population decline was confirmed in the Broughton Archipelago in the fall of 2002, government regulators worked with fish farm companies to fallow a migration pathway during the outmigration months of March, April, and May of 2003 (16, 33). As a result of this voluntary program, three farms in the Tribune-Fife corridor (Fig. S1) that had been stocked in March 2002 were fallowed from March through June 2003. The Knight Inlet corridor (Fig. S1) was not fallowed in 2003, and total numbers of farm fish in the Broughton were about the same in March 2002 (6,717,000), 2003 (7,142,000), and 2004 (7,223,000) (Fig. S5 and Movie S1). The three farms fallowed in 2003 were restocked with young fish by March 2004, and thereafter, the Tribune-Fife corridor was not fallowed. The second option (closed containment) has not been tested at commercial production levels.
The year of coordinated fallowing (2003) was associated with decreased sea lice prevalence on juvenile pink salmon in the fallowed corridor (16) and in the corridor that was not fallowed (34), and sea lice numbers on all Broughton farm salmon were generally less in 2003 than in either 2002 or 2004 (Fig. 1). Among several hypotheses to explain these changes in sea lice prevalence, the strength of the relationship between L. salmonis on wild and farm fish best supports the hypothesis that the decrease in 2003 was a result of the precipitous decline of the parent generation in 2002 (16) and fewer numbers of lice per returning fish. In support, escapement of the pink salmon parent generation was less in 2002 (110,300) than in either 2001 (1,490,000) or 2003 (186,800), and even-year pink salmon populations are associated with significantly fewer lice per fish than odd-year populations (Fig. 3A). Decreased sea lice prevalence was not a result of increased treatment of farm fish, because farm fish received fewer emamectin benzoate treatments during the fallow year (September 2002 through August 2003) than in the preceding or following year, and sea lice numbers on treated fish during the fallow year were less than either the preceding or next year (Fig. 2) (10). Finally, the increase in sea lice prevalence in 2004 and some years thereafter could not have resulted from lack of fallowing after 2003, because the three farms that were fallowed in 2003 hosted almost no sea lice from 2004 to 2007 (Fig. S5). Only in 2008 were farm lice numbers relatively high in the previously fallowed corridor (Fig. S5), when fish treatment at one farm was delayed until March (Dataset S1) because of lack of availability of emamectin benzoate from the manufacturer. In March 2008, this farm hosted 43% of the 1.0 million adult female L. salmonis on all Broughton farm fish, but adult returns (in 2009) for juvenile pink salmon migrating through the Broughton in 2008 were nearly triple that of their parent generation (in 2007) (Fig. S2).
Conclusion
Adult pink salmon returns the previous fall are a good predictor of sea lice prevalence in the spring, but farm sea lice numbers are not a good predictor of wild salmon survival. Indeed, we estimate that farm-source sea lice numbers were greater during juvenile pink salmon outmigration in March 2000 than in March 2001 (9.1 vs. 7.5 million) (Fig. 1), providing no intimation of record high adult returns in 2001 vs. the 97% population collapse in 2002 (Fig. S2). Based on the lack of evidence for a significant negative relationship between farm fish and pink salmon productivity, the data do not support the hypothesis that separating farm fish from wild fish will increase pink salmon marine survival. Determination of the cause(s) of salmon population decline requires investigation of other variables. For example, in 2001, sick juvenile pink salmon frequently had “bleeding at the base of the fins” (4), but this lesion does not occur in pink salmon exposed to Pacific-source L. salmonis under controlled laboratory conditions (20, 32). Instead, reddening of the fins is commonly associated with stressful environmental conditions or bacterial and viral infections (35, 36); however, none of these differentials were investigated in 2001, and their potential role in fish mortality that year remains unknown. Adding medical analysis to multidisciplinary investigations of fish population decline can increase our understanding of the cause and help government agencies develop cost-effective regulations to sustain healthy wild salmon populations.
Methods
To determine the relationship of farm-source sea lice and pink salmon productivity, we obtained data from all salmon farms and major pink salmon rivers in the Broughton Archipelago region of Western Canada. Annual farm fish production (Gg · y−1) data are from 1990 to 2009 (Dataset S1). Monthly farm data (from 2000 to 2009) include: inventory (number of fish per farm), sea lice counts (number of adult female L. salmonis and C. clemensi per fish), and medical treatments for sea lice (Dataset S1). We used these data to estimate the monthly total of adult female sea lice on all farm fish in the Broughton Archipelago from as early as March 2000 (SI Methods), more than 1 y before sea lice were first reported on wild juvenile pink salmon. Data were compared with pink salmon escapement (number of wild salmon that return to a river to spawn in the fall) and commercial harvest data obtained from the Department of Fisheries and Oceans Canada from 1950 to 2009 (Dataset S1).
To test whether returning adult pink salmon could be a source of sea lice on farm fish, we compared adult pink salmon returns (escapement plus commercial harvest) with farm adult female L. salmonis numbers the next April. Separate linear regressions were done for returns to the Broughton Archipelago for all years (1999–2008) and for the even- and odd-year runs separately followed by significance testing of differences in the slope of each run's regression (37). A multiple linear regression was done for returns to the Broughton Archipelago and the nearby Fraser River for all years, with both runs combined.
To test whether sea lice infestation of juvenile pink salmon from 2002 to 2009 is related to the number of L. salmonis on farm fish, we compared farm fish adult female L. salmonis numbers in April with the prevalence of L. salmonis on juvenile pink salmon in mid-May (16, 21, 34, 38, 39). The 1-mo lag accounts for the time needed for a louse egg to develop into an infectious copepodid stage (24). We considered comparing farm lice numbers in March with juvenile pink salmon lice prevalence in April, but we did not do so because pink salmon lice data are not available for April 2002: the year of greatest farm lice numbers during March or April. Visual inspection of the data suggested a curvilinear relationship, and therefore, data were fitted to a von Bertalanffy curve: y = a(1 − e−bx), where y is the L. salmonis prevalence on wild outmigrating juvenile pink salmon, x is the number of adult female farm L. salmonis, a is the maximum prevalence, and b is the rate of increase in prevalence.
To test the relationship between pink salmon returns and farm fish variables, we used a generalization of the classic Ricker spawner–recruit model, which relates returns of one generation to escapement of the previous generation (40). It predicts that returns are a dome-shaped function of the spawning population because of density-dependent effects. The generalized model also includes effects of the farm fish variables. Our approach is similar to a previous study that implicated sea lice in wild salmon declines (3), except that our study uses data from fish farms. We tested the impact of fish farms on pink salmon productivity (in year t) in the Broughton Archipelago using two variables to represent fish farm effects: March adult female L. salmonis numbers (year t − 1) and annual farm fish production (year t − 1). Because total March adult female L. salmonis numbers on farm salmon were not correlated with same year farm fish production from 2000 to 2009 (linear regression, P = 0.99), these variables provide different measures of the potential effects of fish farms on pink salmon. The other variable in the linear regression analysis is escapement 2 y earlier (year t − 2). A complete derivation of this relationship from assumptions about mortality during early life history is described in SI Methods.
Supplementary Material
Acknowledgments
Veterinarians W. Cox, P. Hardy-Smith, G. Karreman, P. McKenzie, B. Milligan, and D. Morrison oversaw sea lice counts. B. Boyce, S. Fukui, L. Jensen, M. Parker, G. Robinson, and P. Wiper compiled farm fish inventory, sea lice counts, and treatment data. Grieg Seafoods BC, Mainstream Canada, and Marine Harvest Canada provided or approved the release of all farm data for publication. From British Columbia offices of the Department of Fisheries and Oceans, Canada, S. Anderson, M. Mortimer, W. Levesque, B. Spencer, P. van Will, and D. Wagner provided pink salmon escapement data; P. van Will provided catch data. From the British Columbia Provincial Government, Canada, S. Cheeseman provided the map, C. Matthews provided farm fish production data, and veterinarians J. Constantine, R. Lewis, A. Osborn, I. Keith, M. Sheppard, and P. Kitching oversaw development and implementation of regulations that mandated consistent generation of farm lice data since 2003. W. Smoker provided advice on analyzing the data. Dianne Marty reviewed the manuscript. G.D.M. appointments include (i) fish pathologist, Ministry of Agriculture, British Columbia Provincial Government, and (ii) affiliate faculty, School of Fisheries and Ocean Sciences, University of Alaska, Fairbanks.
Footnotes
Conflict of interest statement: None of the authors received compensation from any source for this analysis. S.M.S., as part of her private veterinary practice over the past 15 y, has done contract work for all three fish farm companies that operate in the study area (these companies are cited in the acknowledgments, and this relationship was vital for obtaining all proprietary farm medical records for this study); S.M.S.’s spouse started working for closed-containment aquaculture operation in September 2010.
This article is a PNAS Direct Submission. C.W. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009573108/-/DCSupplemental.
References
- 1.Costello MJ. How sea lice from salmon farms may cause wild salmonid declines in Europe and North America and be a threat to fishes elsewhere. Proc R Soc B Biol Sci. 2009;276:3385–3394. doi: 10.1098/rspb.2009.0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krkosek M. Sea lice and salmon in Pacific Canada: Ecology and policy. Front Ecol Environ. 2010;8:201–209. [Google Scholar]
- 3.Krkosek M, et al. Declining wild salmon populations in relation to parasites from farm salmon. Science. 2007;318:1772–1775. doi: 10.1126/science.1148744. [DOI] [PubMed] [Google Scholar]
- 4.Morton AB, Williams R. First report of a sea louse, Lepeophtheirus salmonis, infestation on juvenile pink salmon, Oncorhynchus gorbuscha, in nearshore habitat. Can Field-Nat. 2003;117:634–641. [Google Scholar]
- 5.Heard WR. Life history of pink salmon (Oncorhynchus gorbuscha) In: Groot C, Margolis L, editors. Pacific Salmon Life Histories. Vancouver: University of British Columbia Press; 1991. pp. 119–230. [Google Scholar]
- 6.Kabata Z. The species of Lepeophtheirus (Copepoda: Caligidae) from fishes of British Columbia. J Fish Res Bd Can. 1973;30:729–759. [Google Scholar]
- 7.Beamish RJ, Neville CM, Sweeting RM, Ambers N. Sea lice on adult Pacific salmon in the coastal waters of Central British Columbia, Canada. Fisheries Research. 2005;76:198–208. [Google Scholar]
- 8.Morton A, Williams R. Response of the sea louse Lepeophtheirus salmonis infestation levels on juvenile wild Pink, Oncorhynchus gorbuscha, and Chum, O. keta, salmon to arrival of parasitized wild adult Pink Salmon. Can Field-Nat. 2006;120:199–204. [Google Scholar]
- 9.Krkosek M, et al. Effects of host migration, diversity and aquaculture on sea lice threats to Pacific salmon populations. Proc Biol Sci. 2007;274:3141–3149. doi: 10.1098/rspb.2007.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saksida S, Constantine J, Karreman GA, Donald A. Evaluation of sea lice abundance levels on farmed Atlantic salmon (Salmo salar L.) located in the Broughton Archipelago of British Columbia from 2003 to 2005. Aquacult Res. 2007;38:219–231. [Google Scholar]
- 11.Yazawa R, et al. EST and mitochondrial DNA sequences support a distinct Pacific form of salmon louse, Lepeophtheirus salmonis. Mar Biotechnol (NY) 2008;10:741–749. doi: 10.1007/s10126-008-9112-y. [DOI] [PubMed] [Google Scholar]
- 12.Todd CD, Walker AM, Ritchie MG, Graves JA, Walker AF. Population genetic differentiation of sea lice (Lepeophtheirus salmonis) parasitic on Atlantic and Pacific salmonids: Analyses of microsatellite DNA variation among wild and farmed hosts. Can J Fish Aquat Sci. 2004;61:1176–1190. [Google Scholar]
- 13.Beamish R, et al. A large, natural infection of sea lice on juvenile Pacific salmon in the Gulf Islands area of British Columbia, Canada. Aquaculture. 2009;297:31–37. [Google Scholar]
- 14.Krkosek M, Lewis MA, Morton A, Frazer LN, Volpe JP. Epizootics of wild fish induced by farm fish. Proc Natl Acad Sci USA. 2006;103:15506–15510. doi: 10.1073/pnas.0603525103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orr C. Estimated sea louse egg production from Marine Harvest Canada farmed Atlantic salmon in the Broughton Archipelago, British Columbia, 2003–2004. N Am J Fish Manage. 2007;27:187–197. [Google Scholar]
- 16.Morton A, Routledge RD, Williams R. Temporal patterns of sea louse infestation on wild Pacific salmon in relation to the fallowing of Atlantic salmon farms. N Am J Fish Manage. 2005;25:811–821. [Google Scholar]
- 17.Jones SRM. Controlling salmon lice on farmed salmon and implications for wild salmon. CAB Rev Perspect Ag Vet Sci Nutr Nat Res. 2009;4:1–13. [Google Scholar]
- 18.Krkosek M, Morton A, Volpe JP, Lewis MA. Sea lice and salmon population dynamics: Effects of exposure time for migratory fish. Proc Biol Sci. 2009;276:2819–2828. doi: 10.1098/rspb.2009.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morton A, Routledge R. Mortality rates for juvenile pink Oncorhynchus gorbuscha and chum O. keta salmon infested with sea lice Lepeophtheirus salmonis in the Broughton Archipelago. Alask Fish Res Bull. 2005;11:146–152. [Google Scholar]
- 20.Jones S, Kim E, Bennett W. Early development of resistance to the salmon louse, Lepeophtheirus salmonis (Krøyer), in juvenile pink salmon, Oncorhynchus gorbuscha (Walbaum) J Fish Dis. 2008;31:591–600. doi: 10.1111/j.1365-2761.2008.00933.x. [DOI] [PubMed] [Google Scholar]
- 21.Jones SRM, Hargreaves NB. Infection threshold to estimate Lepeophtheirus salmonis-associated mortality among juvenile pink salmon. Dis Aquat Org. 2009;84:131–137. doi: 10.3354/dao02043. [DOI] [PubMed] [Google Scholar]
- 22.Brett JR, Griffioen W, Solmie A. Nanaimo, British Columbia, Canada: Department of Fisheries and the Environment; 1978. The 1977 crop of salmon reared on the Pacific Biological Station experimental fishfarm. Fisheries and Marine Service Technical Report 845, 17. [Google Scholar]
- 23.Webster SJ, Dill LM, Butterworth K. The effect of sea lice infestation on the salinity preference and energetic expenditure of juvenile pink salmon (Oncorhynchus gorbuscha) Can J Fish Aquat Sci. 2007;64:672–680. [Google Scholar]
- 24.Johnson SC, Albright LJ. Development, growth, and survival of Lepeophtheirus salmonis (copepoda, caligidae) under laboratory conditions. J Mar Biol Assoc UK. 1991;71:425–436. [Google Scholar]
- 25.Nagasawa K. Annual changes in the population size of the salmon louse Lepeophtheirus salmonis (Copepoda: Caligidae) on high-seas Pacific salmon (Oncorhynchus spp.), and relationship to host abundance. Hydrobiologia. 2001;453:411–416. [Google Scholar]
- 26.Krkosek M, Lewis MA, Volpe JP. Transmission dynamics of parasitic sea lice from farm to wild salmon. Proc R Soc B Biol Sci. 2005;272:689–696. doi: 10.1098/rspb.2004.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morton A, Routledge R, Peet C, Ladwig A. Sea lice (Lepeophtheirus salmonis) infection rates on juvenile pink (Oncorhynchus gorbuscha) and chum (Oncorhynchus keta) salmon in the nearshore marine environment of British Columbia, Canada. Can J Fish Aquat Sci. 2004;61:147–157. [Google Scholar]
- 28.Johnson SC, Margolis L. Efficacy of ivermectin for control of the salmon louse Lepeophtheirus salmonis on Atlantic salmon. Dis Aquat Org. 1993;17:101–105. [Google Scholar]
- 29.Hunter JG. Survival and production of pink and chum salmon in a coastal stream. J Fish Res Board Can. 1959;16:835–886. [Google Scholar]
- 30.Parker RR. Marine mortality schedules of pink salmon of Bella Coola River, Central British Columbia. J Fish Res Board Can. 1968;25:757–794. [Google Scholar]
- 31.Cohen J. Fulfilling Koch's postulates. Science. 1994;266:1647. doi: 10.1126/science.7992045. [DOI] [PubMed] [Google Scholar]
- 32.Jones SRM, Fast MD, Johnson SC, Groman DB. Differential rejection of salmon lice by pink and chum salmon: Disease consequences and expression of proinflammatory genes. Dis Aquat Org. 2007;75:229–238. doi: 10.3354/dao075229. [DOI] [PubMed] [Google Scholar]
- 33.Beamish RJ, et al. Exceptional marine survival of pink salmon that entered the marine environment in 2003 suggests that farmed Atlantic salmon and Pacific salmon can coexist successfully in a marine ecosystem on the Pacific coast of Canada. ICES J Mar Sci. 2006;63:1326–1337. [Google Scholar]
- 34.Jones SRM, Nemec A. Pink Salmon Action Plan: Sea Lice on Juvenile Salmon and Some Non-Salmonid Species in the Broughton Archipelago in 2003. Ottawa, ON, Canada: Fisheries and Oceans Canada; 2004. p. 87. Canadian Science Advisory Secretariat Research Document 2004/105. [Google Scholar]
- 35.Noga EJ. Fish Disease Diagnosis and Treatment. Ames, IA: Iowa State University Press; 2000. [Google Scholar]
- 36.Skall HF, Olesen NJ, Mellergaard S. Viral haemorrhagic septicaemia virus in marine fish and its implications for fish farming—a review. J Fish Dis. 2005;28:509–529. doi: 10.1111/j.1365-2761.2005.00654.x. [DOI] [PubMed] [Google Scholar]
- 37.Zar JH. Biostatistical Analysis. 4th Ed. Englewood Cliffs, NJ: Prentice Hall; 1999. [Google Scholar]
- 38.Jones SRM, Hargreaves NB. The abundance and distribution of Lepeophtheirus salmonis (Copepoda: Caligidae) on pink (Oncorhynchus gorbuscha) and chum (O. keta) salmon in coastal British Columbia. J Parasitol. 2007;93:1324–1331. doi: 10.1645/GE-1252.1. [DOI] [PubMed] [Google Scholar]
- 39. Anonymous (2009) Pacific region pink salmon action plan: biweekly results of the marine research project in 2009. Available at http://www.pac.dfo-mpo.gc.ca/science/aquaculture/pinksalmon-saumonrose/results-resultats/2009/09biweekly-bimensuels-eng.htm#April_28_-_May_3,_2009. Accessed November 27, 2010.
- 40.Quinn TJ, II, Deriso RB. Quantitative Fish Dynamics. New York: Oxford University Press; 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.