Figure 3. Deamidated gliadin peptide binds specifically to HLA-DQ2.5-derived RTL800.
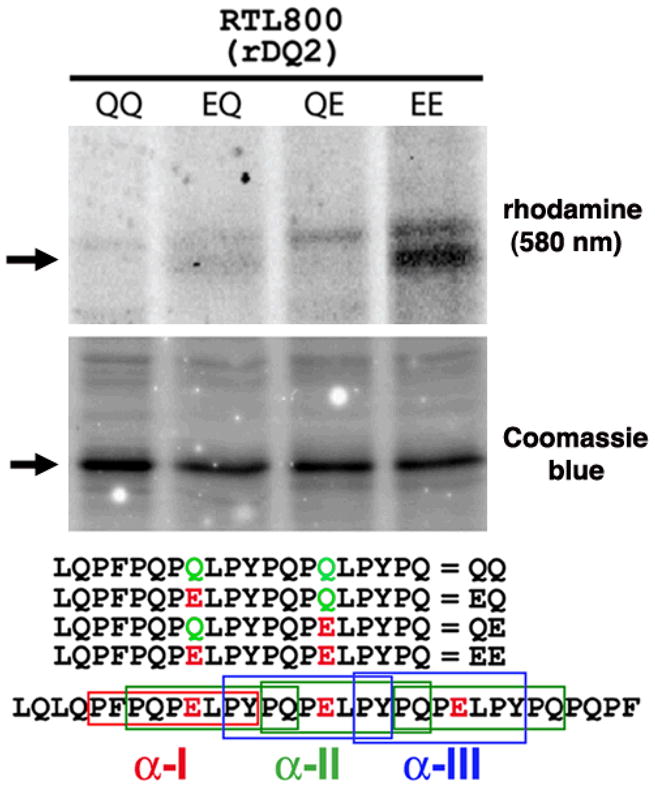
RTLs (20 pMol; ~500 ng) were mixed for 60h at 37 C with 4 nMol of rhodamine-labeled peptides in 100 mM Phosphate, pH 6.0, 0.01% sodium azide and 1 mM EDTA. The reactions were stopped by adding one volume (60 ul) of 1.5 M Tris, 0.1% SDS and 20% glycerol pH 8.8. Forty ul were analyzed by 18% SDS-PAGE and scanned at 580 nm to monitor binding of amino-terminal rhodamine labeled peptides (top panel). Equivalent amounts of RTLs were loaded in each lane, as indicated by staining with Coomassie Blue (middle panel). (Lower panel), the amino-terminal rhodamine-labeled peptides used in this binding study, aligned relative to their position within the immunodominant α2-gliadin-57-89 33-mer. Differences in the sequences of each peptide at key positions are indicated. Boxed regions of the α2-gliadin 33-mer correspond to the α-I (red), α-II (green), and α-III (blue) gliadin minimal epitopes, of which one, three and two copies of each, respectively, are found within the 33-mer peptide. Data are representative of three independent experiments.
