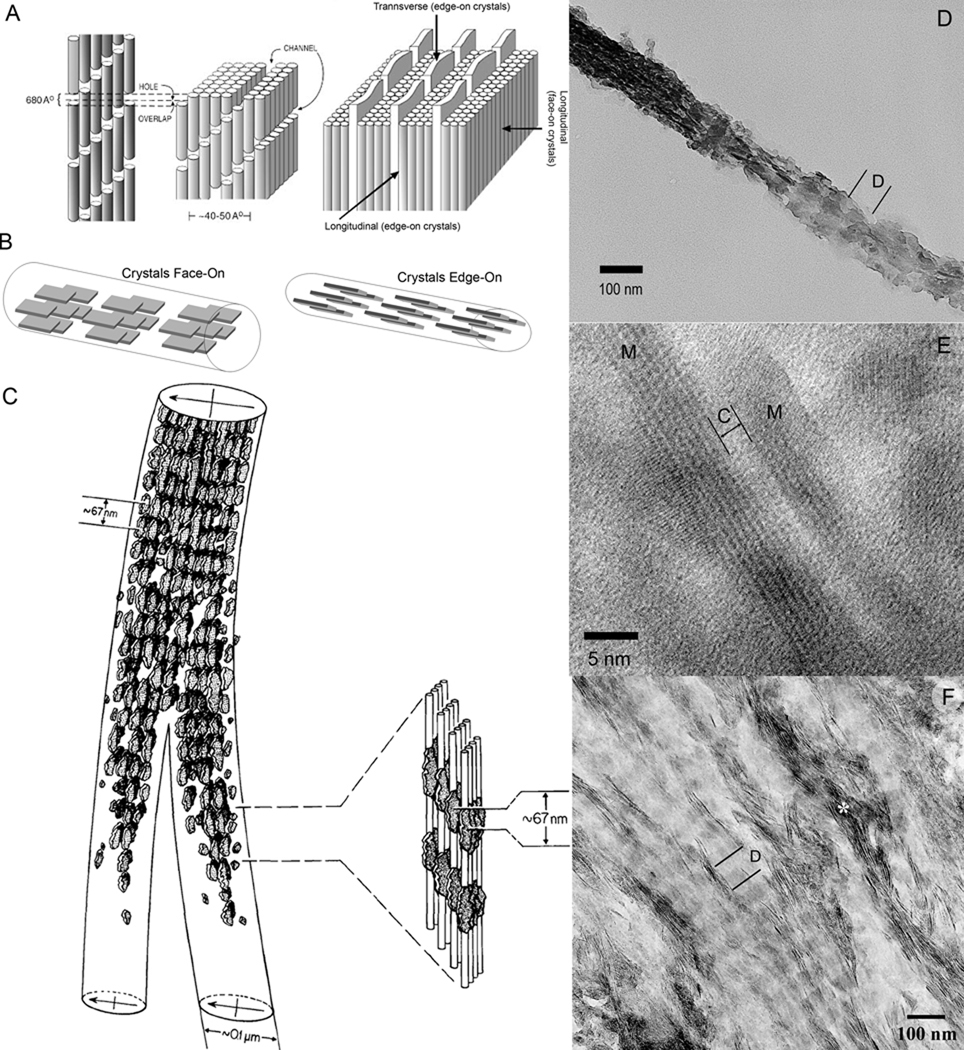
Figure 3.
Structural organization of the mineralized collagen fibril. A. Schematic representation of collagen fibril showing channels in which crystals start to form (left side); on the right, arrays of plate like mineral crystals sandwiched between layers of collagen triple-helices (depicted as cylinders), note that the structure of the mineralized collagen fibril is orthogonally anisotropic. Reprinted with permission from 4 B. Face-on and Edge-on projections of the crystals in the mineralized fibril. C. The drawing of two mineralized collagen fibrils obtained from the electron tomographic reconstruction of the normally calcified mineralized avian tendon. Note that the density of the crystals is higher in the gap regions, leading to a periodic mineral density profile with 67 nm spacing. Also note that the periodic patterns in the neighboring fibrils are in register. Reprinted with permission from 49. D. TEM micrograph of an isolated mineralized collagen fibril from human dentin. The fibril is twisted and both edge-on (left) and face-on (right) crystals present in the same fibril. Note the periodic pattern of mineral density (D-period). E. HRTEM micrograph of two edge-on crystals (M) in the mineralized fibril from human dentin. The narrow space between them is filled with collagen molecules (C). F. An array of the mineralized fibrils from human dentin with crystals in face-on orientation, with the exception of the fibril marked with asterisk with crystals in edge-on orientation. Note the periodic changes in mineral density, corresponding to D-period. Also note that the periodic patterns in neighboring fibrils are in register.