This year marks the 100th year since sickle cell disease (SCD) was first described in Western medical literature by Dr. James B. Herrick, a Chicago physician who published his observations of the irregular shape of a patient’s red blood cells in November 1910.1 The myriad clinical features of this hemolytic anemia result from a deceptively simple amino acid substitution of valine for glutamic acid in the sixth position of the β-subunits of hemoglobin. The result is intracellular polymerization under conditions of hypoxia and dehydration, which triggers the characteristic sickle-shaped erythrocytes that Dr. Herrick first depicted. Since this seminal discovery, we have learnt that SCD is as much a disease of endothelial dysfunction as it is a hemoglobinopathy that triggers erythrocyte polymerization. Oxidative stress, chronic endothelial damage, and hemolysis initiate a cascade of events that result in episodic vaso-occlusion, subsequent ischemia-reperfusion injury, and inflammation.2,3 The clinical phenotype of SCD varies widely, influenced by additional genetic factors, and is characterized by anemia, severe pain and potentially life-threatening complications such as bacterial sepsis, splenic sequestration, acute chest syndrome (pneumonia), stroke and chronic organ damage. These and other manifestations result from chronic hemolysis and intermittent episodes of vascular occlusion that cause both acute and chronic tissue injury and organ dysfunction.2,3 Two articles in this issue of Haematologica explore unique mechanisms leading to organ damage and clinical sequelae in both the transgenic mouse model for SCD (SAD mice)4 and in children with SCD,5 exemplifying the advancements in our knowledge achieved over the last 100 years.
Clinical sub-phenotypes of sickle cell disease associated with hemolytic rate
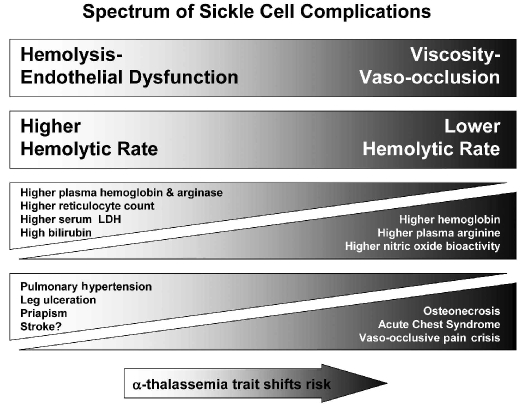
The single sickle mutation is not sufficient to explain the heterogeneity of the disease phenotype that is observed clinically. Two sub-phenotypes of SCD have recently emerged as associated with hemolytic rate: a sub-phenotype of “viscosity - vaso-occlusion” which involves erythrocyte sickling complications such as pain crisis, acute chest syndrome and osteonecrosis, and a second sub-phenotype of “hemolysis -endothelial dysfunction”, a proliferative vasculopathy that involves pulmonary hypertension, priapism, leg ulcers, sudden death, stroke (Figure 1)6,7 and, possibly, asthma.3,8 Although some overlap between the sub-phenotypes is expected, clinical profiling to differentiate symptoms more typical for an individual patient with SCD is an innovative approach that may help direct personalized therapies for a specific sub-phenotype by targeting the predominant mechanism in this multifactorial disorder.
Figure 1.
The spectrum of sickle cell sub-phenotypes affected by hemolytic rate. The viscosity - vaso-occlusion sub-phenotype is associated with a lower hemolytic rate, marked by a higher hemoglobin level, and low plasma hemoglobin, lactate dehydrogenase (LDH), bilirubin and arginase levels. Patients with these features have a higher incidence of vaso-occlusive pain crises, acute chest syndrome, and osteonecrosis. In contrast, patients with the hemolysis - endothelial dysfunction subphenotype exhibit markers of high hemolytic rate, including low hemoglobin level, high plasma hemoglobin, LDH, bilirubin, and arginase, culminating in low nitric oxide bioavailability and high prevalence of pulmonary hypertension, leg ulceration, priapism, and stroke. Co-inheritance of α-thalassemia trait with sickle cell disease reduces the hemolytic rate, minimizes the risk of hemolysis-associated complications and increases the risk of viscosity-related complications. Adapted with permission.6
Mechanisms of vasculopathy
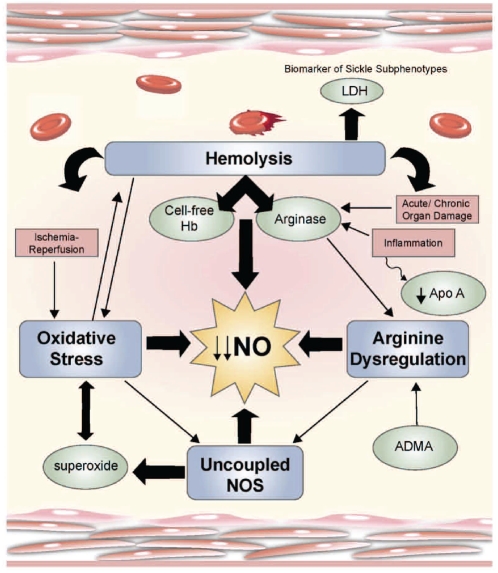
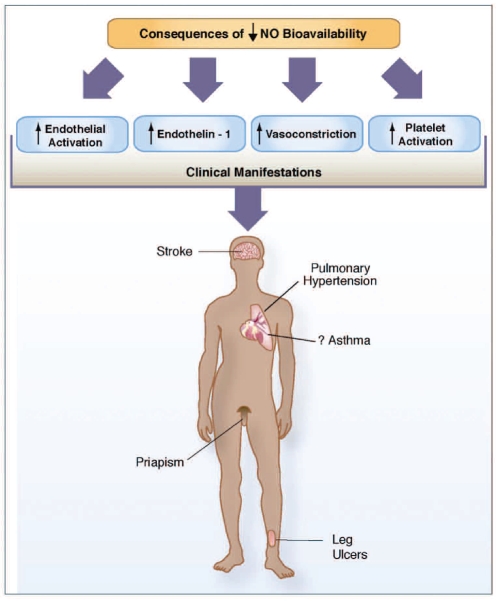
Many mechanisms contribute to the complex pathophysiology of SCD, with dysfunction of the vascular endothelium being a unifying theme (Figure 2).3 Impaired nitric oxide (NO) bioavailability represents the central feature of endothelial dysfunction, and is a common denominator in the pathogenesis of vasculopathy in SCD.6,7 NO is a critical endogenous vasodilator synthesized by endothelial cells from its obligate substrate L-arginine, which is converted to citrulline by a family of enzymes, the NO synthases. The consequences of decreased NO bioavailability include endothelial cell activation, up-regulation of the potent vasoconstrictor endothelin-1, vasoconstriction, platelet activation, increased tissue factor, and activation of coagulation, all of which ultimately translate into the clinical manifestations of SCD (Figure 3).3 The process of hemolysis disrupts the arginine-NO pathway from every angle.3,9–11 Under normal conditions, hemoglobin is safely packaged within the erythrocyte plasma membrane; however, during hemolysis it is decompartmentalized and released into plasma where it rapidly reacts with and destroys NO.12 This results in abnormally high NO consumption and the formation of reactive oxygen species, ultimately inhibiting vasodilatation. The simultaneous release of erythrocyte arginase during hemolysis11 will limit the availability of arginine to NO synthase, contributing to a deficiency of NO. Arginase will redirect the metabolism of arginine away from NO to ornithine and the formation of polyamines and proline, which are essential for smooth muscle cell growth and collagen synthesis. By creating a shift towards ornithine metabolism, arginase likely contributes to the proliferative vasculopathy common to hemolytic disorders,3,11,13 pulmonary hypertension and cardiovascular disease.14 Levels of asymmetric dimethylargi-nine (an arginine analog) are elevated in SCD, associated with hemolysis, pulmonary hypertension and mortality,15 and will also affect global arginine bioavailability.3 Low arginine bioavailability may further compromise NO generation due to NO synthase uncoupling.9,16 Since we first reported an association of low global arginine bioavailability with mortality in SCD,11 low arginine bioavailability has subsequently been linked to mortality risk in both malaria17 and cardiovascular disease in general,14 and represents a novel biomarker of vasculopathy independent of genotype. Intravascular hemolysis also has the potential to drive a pro-coagulant state, as NO has the properties of inhibiting platelet activation, tissue factor expression and thrombin generation.10 While studying the transgenic mouse model for SCD exposed to ischemia/reperfusion stress, Siciliano et al. elucidated additional mechanisms that disrupt NO homeostasis.4 Their results, published in this month’s issue of Haematologica, show that the sickle cell hepatopathy in SAD mice is associated with an imbalance of endothelial/inducible NO synthase and lack of heme oxygenase-1/biliverdin reductase cytoprotection. In addition, up-regulation of phosphodiesterase-4 genes is noted in hepatocytes in response to ischemia/reperfusion stress. Phosphodiesterase-4 is the major cyclic adenosine monophosphate-metabolizing enzyme found in inflammatory and immune cells. Inhibition of phosphodi-esterase-4 by rolipram attenuated ischemia/reperfusion-related vasculopathy and inflammation, while inducing the heme oxygenase-1/biliverdin reductase cytoprotective systems, leading to improved hepatocellular survival.4 The complexity of these mechanisms likely contributes to the varied clinical spectrum of SCD; targeting these pathways may translate into new therapeutic strategies.
Figure 2.
Mechanisms of vasculopathy. Hemolysis, arginine dysregulation, oxidative stress and uncoupled nitric oxide synthase (NOS) are key mechanisms that contribute to the complex vascular pathophysiology of sickle cell disease (SCD). These events limit nitric oxide (NO) bioavailability through several paths that ultimately provoke increased consumption and decreased production of the potent vasodilator, NO. Although often discussed independently, there is significant overlap closely linking these pathways of endothelial dysfunction which prohibit determination of cause and effect. The contribution of inflammation coupled with antioxidant, glutathione and apolipoprotein A-1 (Apo A) depletion, ischemia-reperfusion injury, and acute as well as chronic end-organ damage obscure mechanistic boundaries further. During hemolysis, cell-free hemoglobin and arginase are simultaneously released from the erythrocyte and profoundly contribute to low NO bioavailability. Lactate dehydrogenase (LDH) is also released from the erythrocyte and represents a convenient biomarker of hemolysis that delineates the subphenotypes of sickle cell disease. Reproduced with permission from the American Society of Hematology.3
Figure 3.
Consequences of low nitric oxide bioavailability. The consequences of decreased nitric oxide (NO) bioavailability include endothelial cell activation, up-regulation of the potent vasoconstrictor endothelin-1, vasoconstriction, platelet activation, increased tissue factor and activation of coagulation pathways, all of which ultimately translate into the clinical manifestations of sickle cell disease. NO bioavailability is particularly vulnerable to the effects of hemolysis, an event in sickle cell disease that contributes to the development of the hemolytic sub-phenotypes which include pulmonary hypertension, priapism, cutaneous leg ulceration, stroke and, possibly, asthma. Reproduced with permission from the American Society of Hematology.3
Biomarkers of hemolytic rate
The intensity of hemolysis can be estimated as part of a vascular risk assessment simply by evaluating tertiles or quartiles of plasma lactate dehydrogenase values, as this enzyme is released from erythrocytes along with free hemoglobin and arginase18 and represents a convenient biomarker of intravascular hemolysis that is associated with elevated tricuspid regurgitant jet velocity (TRV), mortality and the hemolytic sub-phenotype of SCD in adults.6 The hemolytic “index” recently described by Minniti et al. is another novel indicator, determined by a principal component analysis of the levels of reticulocyte count, aspartate aminotransferase and bilirubin in addition to lactate dehydrogenase.19
Pulmonary hypertension in adults with sickle cell disease
Although the mechanisms that contribute to the development of pulmonary hypertension in SCD are clearly multifactorial, pulmonary hypertension is the best characterized clinical complication of acute and chronic hemolysis.7 A Doppler echocardiogram-measured TRV of 2.5 m/sec or greater, suggesting a risk of pulmonary hypertension, occurs in about one third of adults20–22 with SCD and is currently the strongest predictor of early death, being associated with an approximately10-fold increased risk of early mortality,20–22 and a 40% mortality risk within 3 years of diagnosis.22 While controversy surrounds the inclusion of a mildly elevated TRV between 2.5–2.9 m/sec in this definition,23,24 this value is approximately two standard deviations greater than normal while a TRV of 3.0 m/s or more is three standard deviations above the mean. A TRV of 2.5 m/sec or greater is also associated with a high risk of death in other conditions, such as congestive heart failure.25 This association between increased TRV and mortality in SCD is significant, and has been confirmed in multiple studies,20,21,26–28 although it remains to be determined whether this measurement and, its association with mortality, reflects true pulmonary hypertension per se, or is a biomarker of disease severity and systemic vasculopathy in SCD. Right heart catheterization studies suggested that 6–11% of adults with SCD have pulmonary hypertension defined by a mean pulmonary artery pressure greater than or equal to 25 mmHg.7,29 Approximately half of these cases are pulmonary artery hypertension, and half are pulmonary venous hypertension.7
Doppler-defined pulmonary hypertension in children with sickle cell disease
The meaning of an elevated TRV is even more obscure in the pediatric population with SCD and is the topic of active research. Early retrospective reports on the prevalence of an elevated TRV in children with SCD were similar to adults.28,30–33 The Pulmonary Hypertension and the Hypoxic Response in Sickle Cell Disease (PUSH) study is currently being conducted to evaluate this population prospectively; an interim analysis of the first 399 SCD patients between the ages of 3 and 20 enrolled suggests a slightly lower prevalence (22%) of TRV elevation above 2.5m/sec.19,34,35 However, based on the mean TRV plus two standard deviations in age- and gender-matched control children, the PUSH study has defined an elevated TRV in children as 2.6 m/sec or above. Using this definition, the prevalence of an elevated TRV is actually 11% in the PUSH study.19 More recently, a study focused on younger, primarily African children (mean age 6.2 years) found a 21.6% prevalence of a TRV of 2.5 m/sec or greater which was associated with a history of acute chest syndrome. Children as young as 3 years old were found to have a TRV of 2.5 m/s or greater. Longitudinal follow-up of eight patients within this cohort revealed persistent and often increasing elevations in TRV suggesting that, even in early childhood, an abnormal echocardiogram can be a useful indicator of a higher risk population.36 A correlation with right heart catheterization data, the gold standard diagnostic test for pulmonary hypertension, is not available for children, apart from in case reports.37 However, the PUSH study demonstrates that an elevated TRV in children is associated with increased hemolytic rate, and oxygen desaturation during a 6-minute walk test.19 Despite a different clinical phenotype described in children with Doppler-defined pulmonary hypertension compared to adults,28 it appears that an elevated TRV may identify children at risk of cardiopulmonary dysfunction.
Mortality: an imperfect outcome measure for children
Although the mortality risk associated with an elevated TRV has been clearly established in adults, the clinical significance of an elevated TRV in children remains unclear. However, no deaths occurred in 15 out of 61 children screened with a TRV of 2.5 m/sec or greater compared to 18 deaths in 81 adults with pulmonary hypertension from a SCD cohort followed over an 8-year period post-echocardiography.28 Recently, data from a prospective study that included 88 children also found no mortality after 3 years of follow-up in 18 subjects with a TRV of 2.5 m/sec or greater.38
These preliminary studies suggest that short-term mortality risk of SCD patients with an elevated TRV may not be as great in children as it is in adults;28,38 however, these children may still be at greater risk of complications in young adulthood and warrant close observation. In addition, more ideal clinical outcome measures for children with SCD are needed. While mortality is an important end-point in adult patients, it may not be the most appropriate outcome for the pediatric population, and should not be considered as the only outcome measure of value for SCD. Quality of life and both short-term and long-term risk of developing other complications of SCD associated with the hemolytic sub-phenotype6 should also be weighed into the equation of considering the clinical importance of an elevated TRV in children. Prospective studies are needed to guide pediatric care, while biomarkers to gauge risk and response to interventions are needed.
In the current issue of Haematologica, Gordeuk et al. respond to this call, adding significantly to the pediatric literature as they report data from the largest prospective screening and longitudinal follow-up cohort study in children with SCD to date, evaluating Doppler echocardiography abnormalities, exercise tolerance and clinical bio-markers.5 The consequences of increased hemolytic rate, elevated TRV and left ventricular dysfunction among 160 children with hemoglobin SS (median age, 13 years; range, 3–20 years) followed for a median of 22 months (range, 9–35 months) are described. A TRV of 2.6 m/s or greater was identified in 14.1% of the 149 participants with a measurable TRV and was more common in children on chronic transfusion therapy. Interestingly, 7.7% of children had increased mitral valve E/Etdi (9.23 or higher), which suggests elevated left ventricular filling pressure. The investigators also noted a 4.4-fold risk of decreased exercise tolerance as depicted by a 10% or greater decline in age-standardized distance covered in the 6-minute walk test at follow-up in children with Doppler-defined pulmonary hypertension, a notable observation given the fact that children typically increase their 6-minute walk distance with age and height escalation. Adding to their previously published data, Gordeuk et al. observed a significant independent association of higher hemolytic component and lower hemoglobin oxygen saturation with a higher TRV both during baseline visits and at repeated observations. A hemolytic component above the mean was associated with a 9-fold increased risk of new onset high TRV over time, and with increased risk of higher follow-up mitral valve E/Etdi. Although both biomarkers were independent predictors of newly developing high TRV, due to the low prevalence of a high E/Etdi, the hemolytic component emerged as the more important risk factor of these two predictors for identifying those at risk of new onset Doppler-defined pulmonary hypertension.5 The role of hemolysis has become a focus of debate in SCD,23,24 however, several cross-sectional studies have identified an association between the degree of hemolysis and pulmonary artery pressures in this hemoglobinopathy.7 This study adds further support to the notion of a hemolytic sub-phenotype in SCD6 which includes children as well as adults.
This work establishes that high baseline values for TRV, mitral valve E/Etdi, and a hemolytic component above the mean were predictive of sustained abnormalities at follow-up, and that Doppler-defined pulmonary hypertension in children was associated with a decline in functional capacity over time. This is consistent with an age-related increase in the prevalence of Doppler-defined pulmonary hypertension linked to a high intensity of hemolysis and to left ventricular diastolic dysfunction in children which parallels findings in adults. Novel biomarkers that identify patients with greater disease severity, such as those at risk of deteriorating exercise capacity, are important. The hemolytic index is, therefore, a significant and novel concept that is of clinical utility for both adults and children with SCD. As in adults, it remains to be determined whether an elevated TRV reflects severity of vasculopathy or true pulmonary hypertension for a subgroup. However these authors provide us with new clinical tools to perform a vascular risk assessment that includes TRV and E/Etdi from Doppler echocardiographic screening, combined with the utilization of a novel hemolytic index. The unique ability of an elevated mitral valve E/Etdi ratio to predict the risk of new acute chest syndrome episodes has not been previously reported, but is also worth additional exploration. Future studies to design a mathematical risk model that utilizes a combination of these clinical and biological biomarkers would have potential synergistic value to create a novel severity index for pediatric SCD.
The data emerging from the PUSH study and others provide additional evidence to suggest that children with a high TRV represent a sicker subpopulation of pediatric SCD patients.5,19 Although a larger cohort followed longitudinally over a longer period is needed to fully determine the clinical significance of hemolysis and Doppler-defined pulmonary hypertension in children with SCD, these children will likely grow up to be adults with an elevated TRV and high hemolytic rate, a group known to be at risk of early mortality.20–22 Younger patients may be more amendable to reversal of pulmonary hypertension when TRV elevations are mild and vascular remodeling is minimal, making early identification paramount. Pioneering research in this field is providing some valuable tools, geared towards vascular risk assessment, which have applications in SCD and beyond.
Footnotes
Financial and other disclosures provided by the author using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are available with the full text of this paper at www.haematologica.org.
References
- 1.Savitt TL, Goldberg MF. Herrick’s 1910 case report of sickle cell anemia. The rest of the story. JAMA. 1989;261(2):266–71. [PubMed] [Google Scholar]
- 2.Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med. 1997;337(11):762–9. doi: 10.1056/NEJM199709113371107. [DOI] [PubMed] [Google Scholar]
- 3.Morris CR. Mechanisms of vasculopathy in sickle cell disease and thalassemia. Hematology Am Soc Hematol Educ Program. 2008:177–85. doi: 10.1182/asheducation-2008.1.177. [DOI] [PubMed] [Google Scholar]
- 4.Siciliano A, Malpeli G, Platt O, Lebouef C, Janin A, Scarpa A, et al. Abnormal modulation of cell protective systems in response to ischemic/reperfusion injury is important in the development of mouse sickle cell hepatopathy. Haematologica. 2011;96(1):24–32. doi: 10.3324/haematol.2010.028506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gordeuk V, Minniti CP, Nouraie M, Campbell AD, Rana S, Luchtman-Jones L, et al. Elevated tricuspid regurgitation velocity and decline in exercise capacity over 22 months of follow up in children and adolescents with sickle cell anemia. Haematologica. 2011;96(1):33–40. doi: 10.3324/haematol.2010.030767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev. 2007;21(1):37–47. doi: 10.1016/j.blre.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gladwin MT, Vichinsky E. Pulmonary complications of sickle cell disease. N Engl J Med. 2008;359(21):2254–65. doi: 10.1056/NEJMra0804411. [DOI] [PubMed] [Google Scholar]
- 8.Field JJ, Stocks J, Kirkham FJ, Rosen CL, Dietzen DJ, Semon T, et al. Airway hyper-responsiveness in children with sickle cell anemia. Chest. 2010 Aug 19; doi: 10.1378/chest.10-1243. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu LL, Champion HC, Campbell-Lee SA, Bivalacqua TJ, Manci EA, Diwan BA, et al. Hemolysis in sickle cell mice causes pulmonary hypertension due to global impairment in nitric oxide bioavailability. Blood. 2007;109(7):3088–98. doi: 10.1182/blood-2006-08-039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293(13):1653–62. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 11.Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, V S, et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension and mortality in sickle cell disease. JAMA. 2005;294(1):81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reiter C, Wang X, Tanus-Santos J, Hogg N, Cannon R, Schechter A, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle cell disease. Nat Med. 2002;8(12):1383–9. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 13.Omodeo-Sale F, Cortelezzi L, Vommaro Z, Scaccabarozzi D, Dondorp AM. Dysregulation of L-arginine metabolism and bioavailability associated to free plasma heme. Am J Physiol Cell Physiol. 2010;299(1):C148–54. doi: 10.1152/ajpcell.00405.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang WHW, Wang Z, Cho L, Brennan DM, Hanzen SL. Diminished global arginine bioavailability and increased arginine catabolism as metabolic profile of increased cardiovascular risk. J Am Coll Cardiol. 2009;53(22):2061–7. doi: 10.1016/j.jacc.2009.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato GJ, Wang Z, Machado RF, Blackwelder WC, Taylor JGT, Hazen SL. Endogenous nitric oxide synthase inhibitors in sickle cell disease: abnormal levels and correlations with pulmonary hypertension, desaturation, haemolysis, organ dysfunction and death. Br J Haematol. 2009;145(4):506–13. doi: 10.1111/j.1365-2141.2009.07658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wood KC, Hebbel RP, Lefer DJ, Granger DN. Critical role of endothelial cell-derived nitric oxide synthase in sickle cell disease-induced microvascular dysfunction. Free Radic Biol Med. 2006;40(8):1443–53. doi: 10.1016/j.freeradbiomed.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 17.Yeo TW, Lampah DA, Tjitra E, Gitawati R, Darcy CJ, Jones C, et al. Increased asymmetric dimethylarginine in severe falciparum malaria: association with impaired nitric oxide bioavailability and fatal outcome. PLoS Pathog. 2010;6(4):e1000868. doi: 10.1371/journal.ppat.1000868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato GJ, McGowan V, Machado RF, Little JA, Taylor Jt, Morris CR, et al. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood. 2006;107(6):2279–85. doi: 10.1182/blood-2005-06-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minniti CP, Sable C, Campbell A, Rana S, Ensing G, Dham N, et al. Elevated tricuspid regurgitant jet velocity in children and adolescents with sickle cell disease: association with hemolysis and hemoglobin oxygen desaturation. Haematologica. 2009;94(3):340–7. doi: 10.3324/haematol.13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ataga KI, Moore CG, Jones S, Olajide O, Strayhorn D, Hinderliter A, et al. Pulmonary hypertension in patients with sickle cell disease: a longitudinal study. Br J Haematol. 2006;134(1):109–15. doi: 10.1111/j.1365-2141.2006.06110.x. [DOI] [PubMed] [Google Scholar]
- 21.De Castro LM, Jonassaint JC, Graham FL, Ashley-Koch A, Telen MJ. Pulmonary hypertension associated with sickle cell disease: clinical and laboratory endpoints and disease outcomes. Am J Hematol. 2008;83(1):19–25. doi: 10.1002/ajh.21058. [DOI] [PubMed] [Google Scholar]
- 22.Gladwin M, Sachdev V, Jison M, Shizukuda Y, Plehn J, Minter K, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350(9):886–95. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 23.Gladwin MT, Barst RJ, Castro OL, Gordeuk VR, Hillery CA, Kato GJ, et al. Pulmonary hypertension and NO in sickle cell. Blood. 2010;116(5):852–4. doi: 10.1182/blood-2010-04-282095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bunn HF, Nathan DG, Dover GJ, Hebbel RP, Platt OS, Rosse WF, et al. Pulmonary hypertension and nitric oxide depletion in sickle cell disease. Blood. 2010;116(5):687–92. doi: 10.1182/blood-2010-02-268193. [DOI] [PubMed] [Google Scholar]
- 25.Farzaneh-Far R, Na B, Whooley MA, Schiller NB. Usefulness of non-invasive estimate of pulmonary vascular resistance to predict mortality, heart failure, and adverse cardiovascular events in Patients With stable coronary artery disease (from the Heart and Soul Study) Am J Cardiol. 2008;101(6):762–6. doi: 10.1016/j.amjcard.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darbari DS, Kple-Faget P, Kwagyan J, Rana S, Gordeuk VR, Castro O. Circumstances of death in adult sickle cell disease patients. Am J Hematol. 2006;81(11):858–63. doi: 10.1002/ajh.20685. [DOI] [PubMed] [Google Scholar]
- 27.Sachdev V, Machado RF, Shizukuda Y, Rao YN, Sidenko S, Ernst I, et al. Diastolic dysfunction is an independent risk factor for death in patients with sickle cell disease. J Am Coll Cardiol. 2007;49(4):472–9. doi: 10.1016/j.jacc.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagar RW, Michlitsch JG, Gardner J, Vichinsky EP, Morris CR. Clinical differences between children and adults with pulmonary hypertension and sickle cell disease. Br J Haematol. 2008;140(1):104–12. doi: 10.1111/j.1365-2141.2007.06822.x. [DOI] [PubMed] [Google Scholar]
- 29.Bachir D, Parent F, Hajji L, Inamo J, Loko G, Lionnet F, et al. Prospective multicentric survey on pulmonary hypertension (PH) in adults with sickle cell disease. Blood. 2009;114 Abstract 572. [Google Scholar]
- 30.Kato GJ, Onyekwere OC, Gladwin MT. Pulmonary hypertension in sickle cell disease: relevance to children. Pediatr Hematol Oncol. 2007;24(3):159–70. doi: 10.1080/08880010601185892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pashankar FD, Carbonella J, Bazzy-Asaad A, Friedman A. Prevalence and risk factors of elevated pulmonary artery pressures in children with sickle cell disease. Pediatrics. 2008;121(4):777–82. doi: 10.1542/peds.2007-0730. [DOI] [PubMed] [Google Scholar]
- 32.Onyekwere OC, Campbell A, Teshome M, Onyeagoro S, Sylvan C, Akintilo A, et al. Pulmonary hypertension in children and adolescents with sickle cell disease. Pediatr Cardiol. 2008;29(2):309–12. doi: 10.1007/s00246-007-9018-x. [DOI] [PubMed] [Google Scholar]
- 33.Ambrusko SJ, Gunawardena S, Sakara A, Windsor B, Lanford L, Michelson P, et al. Elevation of tricuspid regurgitant jet velocity, a marker for pulmonary hypertension in children with sickle cell disease. Pediatr Blood Cancer. 2006;47(7):907–13. doi: 10.1002/pbc.20791. [DOI] [PubMed] [Google Scholar]
- 34.Dham N, Ensing G, Minniti C, Campbell A, Arteta M, Rana S, et al. Prospective echocardiography assessment of pulmonary hypertension and its potential etiologies in children with sickle cell disease. Am J Cardiol. 2009;104(5):713–20. doi: 10.1016/j.amjcard.2009.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gordeuk VR, Campbell A, Rana S, Nouraie M, Niu X, Minniti CP, et al. Relationship of erythropoietin, fetal hemoglobin, and hydroxyurea treatment to tricuspid regurgitation velocity in children with sickle cell disease. Blood. 2009;114(21):4639–44. doi: 10.1182/blood-2009-04-218040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colombatti R, Maschietto N, Varotto E, Grison A, Grazzina N, Meneghello L, et al. Pulmonary hypertension in sickle cell disease children under 10 years of age. Br J Haematol. 2010;150(5):601–9. doi: 10.1111/j.1365-2141.2010.08269.x. [DOI] [PubMed] [Google Scholar]
- 37.Villavicencio K, Ivy D, Cole L, Nuss R. Symptomatic pulmonary hypertension in a child with sickle cell disease. J Pediatr. 2008;152(6):879–81. doi: 10.1016/j.jpeds.2008.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee MT, Small T, Khan MA, Rosenzweig EB, Barst RJ, Brittenham GM. Doppler-defined pulmonary hypertension and the risk of death in children with sickle cell disease followed for a mean of three years. Br J Haematol. 2009;146(4):437–41. doi: 10.1111/j.1365-2141.2009.07779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]