Abstract
Background
Sickle cell disease, a genetic red cell disorder inherited in an autosomal recessive manner, occurs throughout the world. Hepatic dysfunction and liver damage may be present in sickle cell disease, but the pathogenesis of these conditions is only partially understood.
Design and Methods
Transgenic mice with sickle cell disease (SAD mice) and wild-type mice were exposed to an ischemic/reperfusion stress. The following parameters were evaluated: hematologic profile, transaminase and bilirubin levels, liver histopathology, and mRNA levels of nuclear factor-κB p65, endothelial nitric oxide synthase, inducible nitric oxide synthase, heme oxygenase-1 and phosphodiesterase-1, -2, -3, and -4 genes in hepatocytes obtained by laser-capture microdissection. Immunoblotting was used to analyze the expression of the following proteins: nuclear factor-κB p65 and phospho-nuclear factor-κB p65, heme oxygenase-1, biliverdin reductase, heat shock protein-70, heat shock protein-27 and peroxiredoxin-6. A subgroup of SAD mice was treated with the phosphodiesterase-4 inhibitor rolipram (30 mg/Kg/day by gavage) during the ischemic/reperfusion protocol.
Results
In SAD mice the ischemic/reperfusion stress induced liver damage compatible with sickle cell disease hepatopathy, which was associated with: (i) lack of hypoxia-induced nuclear factor-κB p65 activation; (ii) imbalance in the endothelial/inducible nitric oxide synthase response to ischemic/reperfusion stress; (iii) lack of hypoxia-induced increased expression of heme oxygenase-1/biliverdin reductase paralleled by a compensatory increased expression of heat shock proteins 70 and 27 and peroxiredoxin-6; and (iv) up-regulation of the phosphodiesterase-1, -2, -3, and -4 genes. In SAD mice the phosphodiesterase-4 inhibitor rolipram attenuated the ischemic/reperfusion-related microcirculatory dysfunction, reduced the inflammatory cell infiltration and induced the heme oxygenase-1/biliverdin reductase cytoprotective systems.
Conclusions
In SAD mice, sickle cell hepatopathy is associated with perturbed nuclear factor-κB p65 signaling with an imbalance of endothelial/inducible nitric oxide synthase levels, lack of heme oxygenase-1/biliverdin reductase expression and up-regulation of two novel cytoprotective systems: heat shock protein-27 and peroxiredoxin-6.
Keywords: NF-κB p65, endothelial nitric oxide synthase, inducible nitric oxide synthase, heat shock protein-70, heat shock protein-27, peroxiredoxin-6
Introduction
Sickle cell disease (SCD), an autosomal recessive genetic red cell disorder which occurs throughout the world, results from a point mutation (βS, 6V) in codon 6 with the insertion of valine in place of glutamic acid leading to the production of a defective form of hemoglobin called hemoglobin S (HbS).1 Studies of the kinetics of HbS poly-merization following deoxygenation have shown it to be a high order exponential function of hemoglobin concentration, thus highlighting the importance of intracellular HbS concentration in sickling.1,2 Pathophysiological studies have shown that dense, dehydrated red cells play a central role in acute and chronic clinical manifestations of SCD, in which intravascular sickling in capillaries and small vessels leads to vaso-occlusion and impaired blood flow with ischemic cell damage in a variety of organs and tissues.3,4
Hepatic dysfunction and liver damage can occur in SCD.5–11 A review of the literature suggests that ischemic/reperfusion (I/R) injury and a related amplified inflammatory response are of paramount importance in the development of sickle hepatic damage, as in other I/R syndromes (e.g. liver resection, shock and venoocclusive syndromes).5,12,13 Studies in these models of I/R liver injury indicate the important role of the homeostasis of liver microcirculation which is under the control of vasoconstrictive molecules such as endothelin-1 (ET-1) and vasodilatory molecules such as nitric oxide (NO) and carbon monoxide.14 Events that perturb this balanced control result in vasoconstriction of the sinusoidal lumen with reduced local blood flow and tissue oxygenation, further worsened by the entrapment of activated and adherent leukocytes.14,15
Pathophysiological studies have shown that the micro-circulation is critically involved in the pathogenesis of sickle cell organ damage. In addition, the abnormally activated ET-1 system and the reduced NO bioavaibility associated with activated endothelial vascular cells, highly adherent neutrophils and dense, dehydrated sickle red cells, all participate in sickle cell-related tissue injury.4,16,17 Although the liver is not one of the main target organs of SCD, its anatomic organization and function, characterized by a sluggish circulation, high metabolic rate and complex regulation of blood flow in the microcirculation, make this an interesting “window organ” to study the pathogenesis of sickle cell-related tissue damage.
Previous studies on sickle cell mouse models exposed to brief periods of acute hypoxia (1–3 h, 7 to 10% oxygen) to mimic acute sickle cell vaso-occlusive crises have shown up-regulation of nuclear factor-κB (NF-κB), increased oxidative stress, reduced local NO bioavailability and modulation of vaso-active molecules.18-21 Activation of NF-κB has also been shown to be important in other models of I/R liver damage22–25 and recently, Belcher et al. reported functional cross-talk between NF-κB activation and increased expression of heme oxygenase-1 (HO-1), a cytoprotective gene in sickle cell mice.18
We, therefore, exposed a transgenic mouse model for SCD, the SAD mice, to an I/R protocol to study the development of sickle cell hepatopathy. Since previous reports have shown beneficial effects of phosphodiesterase (PDE) inhibitors in different model of I/R liver injury (see Online Supplementary References) we also studied the effects of a PDE-4 inhibitor, rolipram, on the development of mouse sickle cell hepatopathy.
Design and Methods
Animals
Transgenic Hbbs/Hbbs SAD mice (βSAD: βS, βAntilles and βD-Punjab) and C57B6/6J control (wild-type, WT) pathogen-free mice were used. The animals (female and male, 20–25 g in body weight) were aged between 4 and 6 months and free from infectious liver diseases.26 The experiments were carried out in accordance with guidelines from the Italian Ministry of Health and the agreement of the local ethics committee for animal studies.
Ischemic/reperfusion protocol
The I/R injury was induced by hypoxia followed by reoxygenation, as previously reported.26,27 In brief, WT (n=6) and SAD (n=6) mice were evaluated in ambient air and then after hypoxia (8% O2) maintained for 4 h (WT and SAD, n=6), 48 h (WT and SAD, n=6) or 168 h (WT and SAD, n=8), followed by 2 h of reoxygenation. No major problems in mouse behavior or significant changes in mouse weight occurred during the I/R protocol. One group of SAD mice (n=6) was treated with the PDE-4 inhibitor rolipram (Sigma-Aldrich Co, St Louis, MO, USA) at the dose of 30 mg/Kg once a day by gavage. Blood sampling and vehicle administration had been previously shown not to affect the blood parameters measured in this study.26,27 Treatment was started 48 h before hypoxia and maintained during the 168 h of hypoxia. Mice did not show major side effects related to rolipram treatment. In the groups of hypoxic SAD mouse, five of six animals were alive after 48 h of hypoxia and six of eight animals were alive after 168 h of hypoxia, while all WT and rolipram-treated SAD mice were alive and well after 168 h of hypoxia. Mice were given free access to water and food.
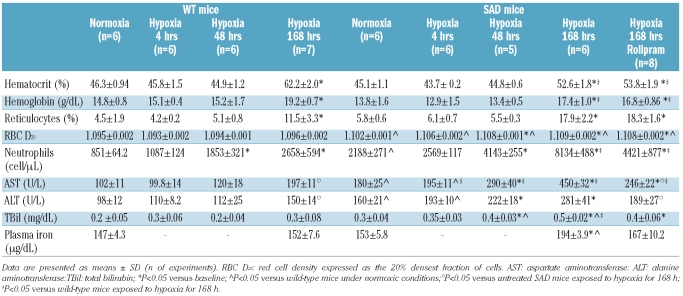
Hematologic parameters were measured at baseline and after the different periods of hypoxia, as previously described.26 Total bilirubin was measured using a Quantichrom Bilirubin Assay kit (BioAssay Systems, Hayward, CA, USA) according to the manufacturer’s instructions.28,29 Plasma iron was measured by flame atomic absorption. Blank reagent was processed in parallel and its iron content was subtracted from that of the samples to correct for background iron.28,30,31 Alanine aminotransferase (ALT) and aspar-tate aminotransferase (AST) liver enzymes were determined using a spectrometric method (Sigma-Aldrich Co, St Louis, MO, USA).32
Histopathology
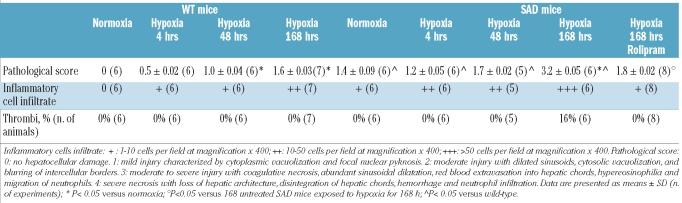
The liver from sacrificed animals was immediately cut into two parts, one of which was frozen immediately in liquid nitrogen, while the other was fixed in formalin and embedded in paraffin. Multiple (at least five) 3 μm whole mount sections were obtained for each paraffin-embedded liver sample and stained with hematoxylin-eosin, Masson’s trichome, and May-Grünwald-Giemsa. A pathological score was determined, as previously described by Duranski et al.32 We also evaluated the inflammatory cell infiltrate and the presence of thrombi. Details on the pathological score are provided in the Online Supplementary Design and Methods.
Molecular studies by quantitative reverse-transcription polymerase chain reaction analysis on hepatocytes
In order to study the effects of I/R stress on gene expression in parenchymal cells, we carried out a molecular analysis using a laser capture microdissection (LCM) approach that allowed us to study hepatocytes as a homogenous cell type.33,34 Hepatocytes identified by cell morphology and isolated by LCM were obtained from frozen liver. LCM was performed on cryostat sections of 8 μm thickness, mounted on 2 μm PEN-membrane coated glass slides (Leica Microsystems, Wetzlar, Germany) and stained with 1:10 diluted hematoxylin (Novocastra, Newcastle upon Tyne, UK). Two hundred hepatocytes from each liver were cut out and collected in a tube using a DM6000 LCM instrument (Leica) and placed immediately in lysis buffer. We examined the tissue section before and after microdissection to verify the homogeneity of the selected cells.33,35 Total RNA was isolated from cells obtained by LCM with an RNAqueous kit (Ambion, Foster City, CA, USA), as suggested by the manufacturer. All RNA samples were retrotran-scribed to cDNA with the High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA). Details on cDNA amplification and quantitative polymerase chain reaction (qPCR) analysis are provided in the Online Supplementary Design and Methods. The oligonucleotide primers used in the qPCR are presented in Online Supplementary Table S1.
Immunoblot analysis
Twenty cryostat sections of 8 μm thickness obtained from frozen liver samples from mice in each studied groups were lysed with iced lysis buffer (containing 150 mM NaCl, 25 mM bicine, 0.1% SDS, Triton 2%, EDTA 1 mM, protease inhibitor cocktail tablets [Roche], 1 mM Na3VO4 final concentration), then centrifuged for 10 min at 4°C at 12,000 g. Proteins were quantified and analyzed by one-dimensional sodium dodecylsulfate poly-acrylamide gel electrophoresis. Gels were transferred to nitrocellulose membranes for immuno-blot analysis with specific antibodies. Details on the antibodies used are provided in the Online Supplementary Design and Methods.
Statistical analysis
A two-way ANOVA algorithm for repeated measures between treatment schedules was used for data analysis. Differences with P values less than 0.05 were considered statistically significant.
Results
Hypoxia/reoxygenation induced sickle cell-related hepatopathy and was associated with a different pattern of nuclear factor-κB expression in SAD mice
In ambient air SAD mice showed mild liver damage characterized by cytoplasmic vacuolization and focal nuclear pyknosis with some dilated sinusoids and inflammatory cell infiltrate associated with increased liver transaminases (Tables 1 and 2). The main foreseen advantages of using young SAD mice were that these animals: (i) did not have an added thalassemic syndrome, and (ii) had only mild liver damage under normoxic conditions. Thus any changes observed during the I/R stress were not obscured by pre-existing pathology, as observed in old SAD mice or in other mouse models of SCD.
Table 1.
Liver pathology of wild-type and SAD mice exposed to hypoxia/reoxygenation and effects of PDE-4 inhibitor (rolipram) treatment.
Table 2.
Hematologic and biochemical parameters in wild-type (WT) and SAD mice exposed to hypoxia/reoxygenation and effects of PDE-4 inhibitor (rolipram) treatment.
The I/R protocol induced a time-dependent worsening of liver damage in the SAD mice with a significant increase in the pathological score after prolonged hypoxia (Table 1). The histological data were consistent with the development of severe liver damage (after 168 h of hypoxia) recapitulating the elements characterizing sickle cell hepatopathy.5,10 In the SAD mice, liver transaminases increased progressively with longer periods of hypoxia, reaching a maximum at 168 h of hypoxia, whereas in the WT mice significant changes in AST and ALT levels were observed only after 168 h of hypoxia (Table 2). In SAD mice, the neutrophil count in the peripheral circulation increased significantly after 4 h of hypoxia and red cell density was maximum after 48 h of hypoxia (Table 2), while in WT mice changes in neutrophil count were present only after 48 h of hypoxia, without modifications of red cell density (Table 2). In the late phase of hypoxia, we observed increased hematocrit, hemoglobin levels and reticulocyte count in both mouse strains, which were changes compatible with the effect of hypoxia on erythropoiesis.27 As we previously reported, prolonged hypoxia also induced a slight worsening of hemolysis in SAD mice, as indicated by the increase in bilirubin and plasma iron levels observed at 168 h of hypoxia only in SAD mice (Table 2).27
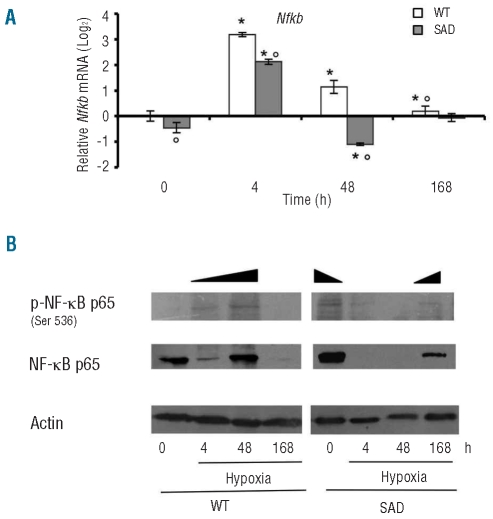
We then evaluated the effects of I/R injury on NF-κB mRNA levels (Nfkb) in laser-captured hepatocytes from both mouse strains. As shown in Figure 1A, in conditions of normoxia, Nfkb mRNA levels were significantly lower in SAD hepatocytes than in WT ones. In the early phase of I/R stress (4 h of hypoxia), we observed increased Nfkb mRNA levels in hepatocytes from both mouse strains independently of the hematologic phenotype, while in the late phase of I/R, Nfkb mRNA levels were down-regulated earlier in SAD hepatocytes than in WT ones, reaching similar values after 168 h of hypoxia in both mouse strains (Figure 1A). We then evaluated the expression of NF-κB p65 and the cellular levels of the active, phosphorylated NF-κB p65 (p-NF-κB p65).36,37 Under normoxia, we observed higher levels of p-NF-κB p65 levels in SAD mouse livers than in WT mouse livers (Figure 1B). I/R stress induced increased levels of p-NF-κB p65 in WT mice at 4 h of hypoxia reaching a peak at 48 h of hypoxia and becoming undetectable at 168 h of hypoxia (Figure 1B). In SAD mice p-NF-κB was undetectable after 4 and 48 h of hypoxia but present at 168 h of hypoxia at a level lower than that observed at baseline but higher than the level in WT mice exposed to the corresponding period of 168 h of hypoxia (Figure 1B).
Figure 1.
(A) Quantitative RT-PCR expression profile of Nfkb, in laser captured hepatocytes from WT mice and SAD mice exposed to I/R stress under normoxia (time 0) and hypoxia (4, 48, and 168 h) followed by 2 h of reoxygenation. Data are presented as means ± SD, n =6–7/group; * P<0.05 compared to baseline values; °P<0.05 compared to WT mice; ^ P< 0.05 compared to untreated SAD mice. The gene expression levels recorded after different experimental conditions were normalized using the average of the expressions of Gapdh and rRNA18s as an endogenous reference. Data were calculated by the comparative method. (B) Immunoblot analysis with specific anti-phospho-NF-κB p65 and anti-NF-κB p65 antibody of hepatocytes from WT and SAD mice under normoxia (time 0) and hypoxia (4, 48, and 168 h) followed by 2 h of reoxygenation. A representative experiment of six performed with similar results is shown.
Since the transcriptional factor NF-κB p65 has been shown to be important in modulation of expression of cytoprotective genes such as endothelial NO synthase (eNOS), inducible NO synthase (iNOS) and HO-1 in other models of ischemic liver injury,38–41 we then evaluated the expression of these genes in livers during the development of sickle cell hepatopathy.
Abnormal modulation of endothelial nitric oxide synthase, heme oxygenase-1 and biliverdin reductase in response to ischemic/reperfusion stress in SAD mouse hepatocytes
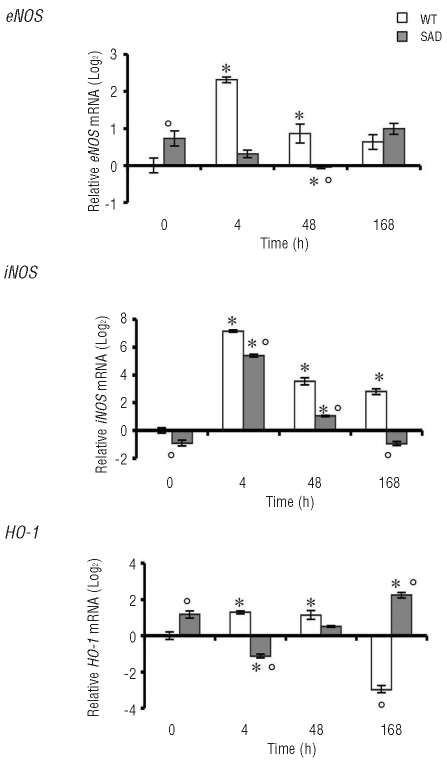
Under normoxic condition the SAD mouse hepatocytes showed up-regulation of eNOS and HO-1 compared to WT mice, associated with mild liver damage as a possible response to the chronic oxidative stress related to the sickle cell phenotype (Figure 2). In the early phase of hypoxia eNOS mRNA levels were down-regulated in SAD mice, going back to baseline levels after prolonged hypoxia, whereas, in WT mice, eNOS mRNA levels were markedly up-regulated in the early phase of I/R stress and then down-regulated to values similar to those observed in SAD mice but still higher than those observed in WT mice under normoxic conditions (Figure 2). iNOS mRNA levels were similarly up-regulated in response to I/R stress in both mouse strains (Figure 2). However, hepatocyte iNOS expression was down-regulated earlier in SAD mice than in WT mice, indicating a perturbed eNOS/iNOS response to I/R stress in SAD mouse hepatocytes (Figure 2).
Figure 2.
Quantitative RT-PCR expression profile of eNOS (endothelial NO synthase), iNOS (inducible NO synthase), and HO-1 (heme oxygenase-1), in laser-captured hepatocytes from WT mice and SAD mice under normoxia (time 0) and hypoxia (4, 48, and 168 h) followed by 2 h of reoxygenation. Data are presented as means±SD, n =6–7 mice/group; * P<0.05 compared to baseline values; °P<0.05 compared to WT mice. For each gene, the expression levels found after different experimental conditions were normalized using the average of the expressions of Gapdh and rRNA18s as an endogenous reference. Data were calculated by the comparative method.
We then evaluated HO-1 mRNA levels in hepatocytes from both mouse strains at the different time points. As reported by Belcher et al. under normoxic conditions, HO-1 mRNA levels were up-regulated in SAD mouse livers compared to WT ones (Figure 2). However, in SAD mice I/R stress induced HO-1 down-regulation in the early phase of hypoxia followed by up-regulation of HO-1 after prolonged hypoxia (168 h) (Figure 2). Contrariwise, in WT mice, HO-1 mRNA levels were significantly increased in the early phase of I/R stress and down-regulated in the late phase of I/R damage (Figure 2).
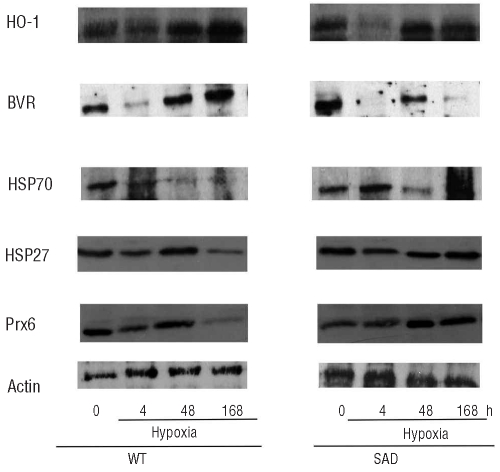
HO-1 protein expression in response to I/R stress was significantly higher in SAD mouse liver than in WT mouse liver (Figure 3). In the early phase of I/R stress we observed a significant reduction in HO-1 protein expression in SAD mice compared to that in either SAD mice at baseline or control mice after the same period of hypoxia (Figure 3). At 48 h of hypoxia, HO-1 protein expression in SAD mice was increased to values similar to those observed at baseline, which were maintained at 168 h of hypoxia in the presence of sickle cell hepatopathy. In WT mice HO-1 protein expression was reduced after 4 h of hypoxia and then increased, such that its levels were higher at 48 and 168 h than either baseline levels or levels in SAD mice (Figure 3).
Figure 3.
Immunoblot analysis of heme oxygenase-1 (HO-1), biliverdin reductase (BVR), heat shock protein 70 (HSP70), heat shock protein 27 (HSP27) and peroxiredoxin-6 (Prx6) expression in livers from WT and SAD mice under normoxia (time 0) and hypoxia (4, 48, and 168 h) followed by 2 h of reoxygenation. A representative experiment of six performed with similar results is shown. Expression of actin was used as a protein-loading control.
In the light of previous reports showing a functional link between HO-1 and biliverdin reductase (BVR) (see Online Supplementary References), we evaluated BVR protein expression in SAD mouse livers under steady state and during I/R stress. In normoxic conditions, as observed for HO-1, BVR protein expression was significantly increased in SAD mice compared to in WT animals (Figure 3). During I/R stress, BVR expression was reduced early (at 4 h) of hypoxia in both mouse strains but then markedly increased in WT mice but not in SAD mice in which the I/R liver damage was more severe.
We then examined whether the perturbations in HO-1 and BVR responses to I/R also occurred in other cytoprotective systems such as the heat shock proteins (HSP), which have been shown to cross-talk with NF-κB, to parallel HO-1 expression and to increase in response to I/R stress in other models of ischemic liver damage (see Online Supplementary References). We evaluated the expression of HSP70 and HSP27 in mouse livers from both mouse strains exposed to I/R stress. We observed similar expression of HSP70 in SAD and control mouse livers under normoxia (Figure 3). I/R induced increased HSP70 expression in the early phase of hypoxia (at 4 h) in both mouse strains, and the levels of expression were similarly reduced after 48 h of hypoxia (Figure 3). In SAD mice, the prolonged I/R stress induced a further increase in HSP70 expression, while WT mice showed a marked reduction of HSP70 levels at 168 h of hypoxia compared to the levels of either normoxic WT mice or SAD mice exposed to hypoxia for 168 h (Figure 3). HSP27 expression was reduced at 4 and 168 h of hypoxia in WT mice, while no significant differences were observed in HSP27 expression in SAD mice exposed to I/R stress. However, the relative increased expression of HSP27 in SAD mice after 168 h of hypoxia was significantly different to that in the group of WT mice exposed to hypoxia for the same period (Figure 3).
Previous in vitro and in vivo studies demonstrated the hepatoprotective effects of Prx6 in different models of liver injury caused by oxidative insults or I/R stress (see Online Supplementary References). In this study we showed that Prx6 expression in normoxic conditions was lower in SAD mice than in WT ones (Figure 3). After 4 h of hypoxia, Prx6 expression was significantly decreased in WT mice, while no changes occurred in the SAD mouse group (Figure 3). After 48 h of hypoxia, Prx6 expression in WT mice increased, reaching values similar to those observed at baseline, while Prx6 expression in SAD mice was significantly increased compared to that in either normoxic SAD mice or WT mice after 48 h of hypoxia. In the SAD mice, prolonged hypoxia (168 h) caused a significant increase in Prx6 expression compared to the level present in normoxic animals, while in the WT mice, the prolonged hypoxia markedly reduced Prx6 expression compared to both baseline values and levels after other shorter periods of hypoxia (Figure 3).
Since previous studies in other models of hepatic I/R injury showed possible beneficial effects of PDE inhibitors42 (see Online Supplementary References) we first evaluated the expression of PDE genes in both mouse strains under normoxia and I/R stress and then administered the PDE-4 inhibitor rolipram to compared the effects of treatment on hepatopathy induced by prolonged hypoxia in SAD mice.
Ischemia/reperfusion induced up-regulation of the expression of genes for phosphodiesterase-1, -2, -3 and -4 isoforms
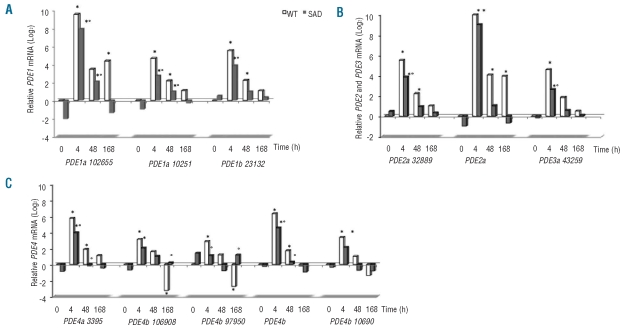
Since different PDE isoforms are involved in hydrolyzation of cyclic nucleotides with overlapping effects, we evaluated the expression of the PDE-1, -2, and -3 families which hydrolyze both cAMP and cGMP as substrates but with different affinities, and the PDE-4 family that hydrolyzes cAMP.27 cAMP and cGMP, acting as second messengers in response to extracellular stimuli, are important in the regulation of vascular tone and in the modulation of neutrophil chemotaxis.27 The following isoforms were undetectable in hepatocytes from both mouse strains: PDE3a (111839), PDE4a (115458-69577-39413), PDE4b (106911), PDE4c (34307-110095) and PDE4d (74103-79975). Some differences in PDE response to hypoxia were observed between WT and SAD mice (Figure 4). Of interest all PDE-4 isoforms were up-regulated in early hypoxia and returned to baseline values in both WT and SAD mice after prolonged hypoxia (Figure 4C).
Figure 4.
(A,B,C) Effects of the ischemic/reperfusion protocol (I/R) on the expression of phosphodiesterase (PDE) 1, 2, 3, 4 gene isoforms in hepatocytes from WT (gray bars) and transgenic SAD mice (black bars). Baseline values under room air condition (time 0), at 4, 48 and 168 h hypoxia followed by 2 h of reoxygenation. Data are reported as median (n=6 mice/groups). For each gene, the expression levels obtained from different experimental conditions were normalized using the average of the expressions of Gapdh and rRNA 18S as an endogenous reference. Data were calculated by the comparative method.
Based on these observations, we administrated an inhibitor of PDE-4 isoforms (rolipram) to SAD mice that developed sickle cell hepatopathy, corresponding to SAD mice exposed to hypoxia for 168 h.
Rolipram has beneficial effects on sickle cell hepatopathy and modulates cytoprotective systems
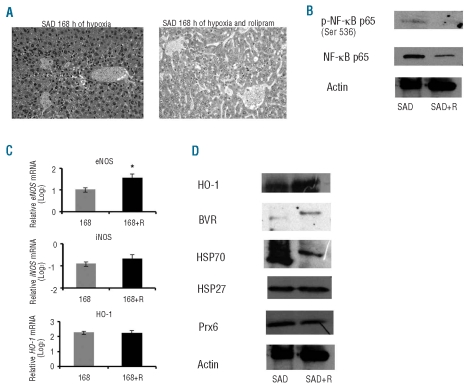
SAD mice treated with the PDE-4 inhibitor rolipram and then exposed to I/R stress showed a significant reduction of liver pathological score, serum liver transaminases and liver inflammatory cell infiltrates compared to either untreated hypoxic SAD mice or hypoxic WT mice (Table 1, Figure 5A). We also observed a significant reduction in total neutrophil counts in these mice compared to untreated hypoxic SAD mice, suggesting a systemic anti-inflammatory effect of the PDE-4 inhibitor (Table 2).
Figure 5.
(A) Representative examples of SAD mouse liver after 168 h of hypoxia with or without the PDE-4 inhibitor rolipram (see also Table 1). (B) Immunoblot analysis with specific anti-phospho-NF-κB p65 and anti-NF-κB p65 antibody of livers from SAD mice exposed to 168 h of hypoxia followed by 2 h of reoxygenation with and without the PDE-4 inhibitor rolipram (SAD+R). Expression of actin was used as a loading control protein. A representative experiment of six performed with similar results is shown. (C) Quantitative RT-PCR expression profile of eNOS (endothelial NO synthase), iNOS (inducible NO synthase), HO-1 (heme oxygenase-1), in laser-captured hepatocytes from SAD mice exposed to 168 h of hypoxia with or without rolipram (168+R) followed by 2 h of reoxygenation. Data are presented as means±SD, n=6–7 mice/group; *P<0.05 compared to untreated SAD mice. For each gene, the expression levels measured after different experimental conditions were normalized using the average of the expressions of Gapdh and rRNA 18S as an endogenous reference. Data were calculated by the comparative method. (D) Immunoblot analysis of heme oxygenase-1 (HO-1), biliverdin reductase (BVR), heat shock protein 70 (HSP70), heat shock protein 27 (HSP27) and peroxiredoxin-6 (Prx6) expression in livers from SAD mice exposed to hypoxia with or without rolipram (SAD+R) followed by 2 h of reoxygenation. A representative experiment of six performed with similar results is shown.
The improvement of sickle cell-related liver damage in SAD mice treated with rolipram compared to the damage in untreated hypoxic SAD mice was also associated with: (i) reduced NF-κB p65 activation; (ii) increased eNOS mRNA levels, and (iii) increased HO-1 and BVR protein expression associated with marked reduction in HSP70 expression and no changes in HSP27 and Prx6 proteins levels (Figure 5B-D).
Discussion
Here we show that normoxic SAD mice have mild, chronic hepatopathy associated with increased cellular levels of p-NF-κB p65 in hepatocytes and up-regulation of cytoprotective genes such as eNOS and HO-1 together with increased HO-1/BVR protein expression possibly induced to limit the liver damage but most likely insufficient to fully counterattack the chronic damage related to SCD.14,43,44 Other protective stress-response systems, such as the molecular chaperones HSP70 and HSP27 and the endogenous anti-oxidant Prx6 (see Online Supplementary References), were similarly expressed in both mouse strains in conditions of normoxia, suggesting that these two HSP and Prx6 are not involved in protecting murine liver cells against chronic sickle cell-related damage.
In SAD mice exposed to I/R stress to induce sickle cell hepatopathy we studied the molecular mechanisms involved in the liver damage of SCD. The development of severe sickle cell hepatopathy was characterized by temporal differences in NF-κB p65 activation in SAD mice compared to in WT mice in response to I/R stress. Previous studies in other models of liver injury showed the important role of NF-κB p65 in signal transduction towards cytoprotective genes such as eNOS, iNOS and HO-1.38,45,46 In SAD mice, we observed a lack in hypoxia-induced eNOS expression in the early phase of I/R stress compared to that in WT mice while the pattern of iNOS expression in response to I/R stress was similar in both mouse strains. Previous studies have shown the importance of controlled balance between iNOS/eNOS levels in local NO homeostasis and microvascular tone regulation to reduce the I/R liver injury.47,48 In SAD mice the imbalance between iNOS/eNOS expression in response to I/R stress may unfavorably affect NO bioavailability, contributing to the dysfunctional liver microcirculation involved in the development of sickle hepatopathy (Figure 3). In addition, the response of other cytoprotective systems, such as HO-1, BVR, HSP and Prx6, to I/R stress also differs between SAD mice and WT ones, representing a new additional factor possibly increasing the susceptibility of the liver of SAD mice to I/R stress. The lack of HO-1 and BVR up-regulation in response to I/R stress observed in SAD mice might contribute to the development of more severe cellular damage beside the biphasic increase of HSP70, which seems not to be sufficient to counteract the sickle cell-related acute organ injury (Figure 5). In SAD mice, expression of both HSP27 and Prx6 was increased after prolonged hypoxia when compared with that in WT mice, which showed significantly reduced expression of both proteins. Although HSP27 and Prx6 are members of different functional groups (molecular chaperones and anti-oxidant systems, respectively), they are both induced in response to oxidative stress in other cell types and their low expression increases cell susceptibility to oxidative stress49 (see also Online Supplementary References). Our data, supported by previously published evidence, indicate a possible novel hepatoprotective role of HSP27 and Prx6 in SAD mice exposed to prolonged I/R stress.
Previous studies in various models of I/R-induced liver injury have shown beneficial effects of PDE inhibitors50 (see Online Supplementary References). In this study we showed that PDE-1, -2, -3, and -4 were up-regulated in the early phase of hypoxia (4 h) in both mouse strains, indicating that these PDE isoforms are involved in the cellular response to I/R cell injury. Blocking the PDE-4 family of proteins by rolipram allowed SAD mice to survive prolonged hypoxia and improved the sickle cell-related liver injury as indicted by: (i) the low liver pathological score; (ii) the marked decrease in local and systemic cell inflammatory response; (iii) the up-regulation of eNOS gene expression balancing iNOS expression; and (iv) the increased HO-1/BVR expression with reduced HSP70 levels. These data suggest that in SAD mice the inhibition of PDE-4 attenuates the microcirculatory dysfunction related to I/R stress, reduces the inflammatory cell infiltration and activates the HO-1/BVR cyto-protective systems, which in turn reduces NF-κB p65 activation with improvement of hepatocellular survival. Further studies should be carried out to better elucidate the functional networks activated in response to I/R stress and involved in the pathogenesis of organ damage in SCD since these may be possible targets for the development of new therapeutic strategies.
Acknowledgments
we thank Prof. Giovanna Fattovich for helpful discussion.
Footnotes
Funding: the research was supported by PRIN and the University of Verona (LDF); and the University of Verona and ARC-NET Research Center (AS).
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Nagel RL, Platt OS. Pathophysiology of sickle cell disease. Disorders of Haemoglobin: Genetics, Pathophysiology, Clinical Management. In: Steinberg MH, Forget BG, Higgs DR, editors. Cambridge, UK: Cambridge University Press; 2001. pp. 494–526. [Google Scholar]
- 2.Eaton WA, Hofrichter J. Sickle cell hemoglobin polymerization. Adv Protein Chem. 1990;40:63–279. doi: 10.1016/s0065-3233(08)60287-9. [DOI] [PubMed] [Google Scholar]
- 3.Solovey AA, Solovey AN, Harkness J, Hebbel RP. Modulation of endothelial cell activation in sickle cell disease: a pilot study. Blood. 2001;97(7):1937–41. doi: 10.1182/blood.v97.7.1937. [DOI] [PubMed] [Google Scholar]
- 4.Hebbel RP. Adhesion of sickle red cells to endothelium: myths and future directions. Transfus Clin Biol. 2008;15(1–2):14–8. doi: 10.1016/j.tracli.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee S, Owen C, Chopra S. Sickle cell hepatopathy. Hepatology. 2001;33(5):1021–8. doi: 10.1053/jhep.2001.24114. [DOI] [PubMed] [Google Scholar]
- 6.Traina F, Jorge SG, Yamanaka A, de Meirelles LR, Costa FF, Saad ST. Chronic liver abnormalities in sickle cell disease: a clinicopathological study in 70 living patients. Acta Haematol. 2007;118(3):129–35. doi: 10.1159/000107744. [DOI] [PubMed] [Google Scholar]
- 7.Charlotte F, Bachir D, Nenert M, Mavier P, Galacteros F, Dhumeaux D, et al. Vascular lesions of the liver in sickle cell disease. A clinicopathological study in 26 living patients. Arch Pathol Lab Med. 1995;119 (1):46–52. [PubMed] [Google Scholar]
- 8.Mills LR, Mwakyusa D, Milner PF. Histopathologic features of liver biopsy specimens in sickle cell disease. Arch Pathol Lab Med. 1988;112(3):290–4. [PubMed] [Google Scholar]
- 9.Bauer TW, Moore GW, Hutchins GM. The liver in sickle cell disease. A clinicopathologic study of 70 patients. Am J Med. 1980;69(6):833–7. doi: 10.1016/s0002-9343(80)80008-8. [DOI] [PubMed] [Google Scholar]
- 10.Sheehy TW. Sickle cell hepatopathy. South Med J. 1977;70(5):533–8. doi: 10.1097/00007611-197705000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Berry PA, Cross TJ, Thein SL, Portmann BC, Wendon JA, Karani JB, et al. Hepatic dysfunction in sickle cell disease: a new system of classification based on global assessment. Clin Gastroenterol Hepatol. 2007;5(12):1469–76. doi: 10.1016/j.cgh.2007.08.009. quiz 369. [DOI] [PubMed] [Google Scholar]
- 12.Teoh NC, Farrell GC. Hepatic ischemia reperfusion injury: pathogenic mechanisms and basis for hepatoprotection. J Gastroenterol Hepatol. 2003;18(8):891–902. doi: 10.1046/j.1440-1746.2003.03056.x. [DOI] [PubMed] [Google Scholar]
- 13.Bynum TE, Boitnott JK, Maddrey WC. Ischemic hepatitis. Dig Dis Sci. 1979;24(2):129–35. doi: 10.1007/BF01324740. [DOI] [PubMed] [Google Scholar]
- 14.Vollmar B, Menger MD. The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol Rev. 2009;89(4):1269–339. doi: 10.1152/physrev.00027.2008. [DOI] [PubMed] [Google Scholar]
- 15.Menger MD, Richter S, Yamauchi J, Vollmar B. Role of microcirculation in hepatic ischemia/reperfusion injury. Hepatogastroenterology. 1999;46 (Suppl 2):1452–7. [PubMed] [Google Scholar]
- 16.Hidalgo A, Chang J, Jang JE, Peired AJ, Chiang EY, Frenette PS. Heterotypic interactions enabled by polarized neutrophil microdomains mediate thromboinflammatory injury. Nat Med. 2009;15(4):384–91. doi: 10.1038/nm.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabaa N, de Franceschi L, Bonnin P, Castier Y, Malpeli G, Debbabi H, et al. Endothelin receptor antagonism prevents hypoxia-induced mortality and morbidity in a mouse model of sickle-cell disease. J Clin Invest. 2008;118(5):1924–33. doi: 10.1172/JCI33308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belcher JD, Mahaseth H, Welch TE, Otterbein LE, Hebbel RP, Vercellotti GM. Heme oxygenase-1 is a modulator of inflammation and vaso-occlusion in transgenic sickle mice. J Clin Invest. 2006;116(3):808–16. doi: 10.1172/JCI26857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belcher JD, Mahaseth H, Welch TE, Vilback AE, Sonbol KM, Kalambur VS, et al. Critical role of endothelial cell activation in hypoxia-induced vasoocclusion in transgenic sickle mice. Am J Physiol Heart Circ Physiol. 2005;288(6):H2715–25. doi: 10.1152/ajpheart.00986.2004. [DOI] [PubMed] [Google Scholar]
- 20.Belcher JD, Mahaseth H, Kalambur VS, Welch TE, Swanlund DJ, Ma L, et al. Microvascular blood flow in transgenic sickle mice: prevention of stasis with polynitroxylalbumin. Sickle Cell Meeting. 2004:19. [Google Scholar]
- 21.Dasgupta T, Hebbel RP, Kaul DK. Protective effect of arginine on oxidative stress in transgenic sickle mouse models. Free Radic Biol Med. 2006;41(12):1771–80. doi: 10.1016/j.freeradbiomed.2006.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huber N, Sakai N, Eismann T, Shin T, Kuboki S, Blanchard J, et al. Age-related decrease in proteasome expression contributes to defective nuclear factor-kappaB activation during hepatic ischemia/reperfusion. Hepatology. 2009;49(5):1718–28. doi: 10.1002/hep.22840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuboki S, Okaya T, Schuster R, Blanchard J, Denenberg A, Wong HR, et al. Hepatocyte NF-kappaB activation is hepatoprotective during ischemia-reperfusion injury and is augmented by ischemic hypothermia. Am J Physiol Gastrointest Liver Physiol. 2007;292(1):G201–7. doi: 10.1152/ajpgi.00186.2006. [DOI] [PubMed] [Google Scholar]
- 24.Shin T, Kuboki S, Lentsch AB. Roles of nuclear factor-kappaB in postischemic liver. Hepatol Res. 2008;38(5):429–40. doi: 10.1111/j.1872-034X.2007.00303.x. [DOI] [PubMed] [Google Scholar]
- 25.Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995;376 (6536):167–70. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 26.de Franceschi L, Baron A, Scarpa A, Adrie C, Janin A, Barbi S, et al. Inhaled nitric oxide protects transgenic SAD mice from sickle cell disease-specific lung injury induced by hypoxia/reoxygenation. Blood. 2003;102(3):1087–96. doi: 10.1182/blood-2002-07-2135. [DOI] [PubMed] [Google Scholar]
- 27.De Franceschi L, Platt OS, Malpeli G, Janin A, Scarpa A, Leboeuf C, et al. Protective effects of phosphodiesterase-4 (PDE-4) inhibition in the early phase of pulmonary arterial hypertension in transgenic sickle cell mice. FASEB J. 2008;22(6):1849–60. doi: 10.1096/fj.07-098921. [DOI] [PubMed] [Google Scholar]
- 28.de Franceschi L, Turrini F, Honczarenko M, Ayi K, Rivera A, Fleming MD, et al. In vivo reduction of erythrocyte oxidant stress in a murine model of beta-thalassemia. Haematologica. 2004;89(11):1287–98. [PubMed] [Google Scholar]
- 29.Vera T, Stec DE. Moderate hyperbilirubine-mia improves renal hemodynamics in ANG II-dependent hypertension. Am J Physiol Regul Integr Comp Physiol. 2010;299(4):R1044–9. doi: 10.1152/ajpregu.00316.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desaulniers JAH, Sturgeon R, Berman SS. Atomic absorption determination of trace metals in marine sediments and biological tissue using a stabilized temperature platform furnace. Atomic Spectroscopy. 1985;6(5):125–7. [Google Scholar]
- 31.Miller-Ihli NJ. Graphite furnace atomic absorption spectrometry for the analysis of biological materials. Spectrochimica Acta. 1989;44(12):1221–7. [Google Scholar]
- 32.Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, et al. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115(5):1232–40. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Espina V, Wulfkuhle JD, Calvert VS, VanMeter A, Zhou W, Coukos G, et al. Laser-capture microdissection. Nat Protoc. 2006;1(2):586–603. doi: 10.1038/nprot.2006.85. [DOI] [PubMed] [Google Scholar]
- 34.Caretti E, Devarajan K, Coudry R, Ross E, Clapper ML, Cooper HS, et al. Comparison of RNA amplification methods and chip platforms for microarray analysis of samples processed by laser capture microdissection. J Cell Biochem. 2008;103(2):556–63. doi: 10.1002/jcb.21426. [DOI] [PubMed] [Google Scholar]
- 35.Chimenti C, Pieroni M, Russo A, Sale P, Russo MA, Maseri A, et al. Laser microdissection in clinical cardiovascular research. Chest. 2005;128(4):2876–81. doi: 10.1378/chest.128.4.2876. [DOI] [PubMed] [Google Scholar]
- 36.Vermeulen L, De Wilde G, Notebaert S, Vanden Berghe W, Haegeman G. Regulation of the transcriptional activity of the nuclear factor-kappaB p65 subunit. Biochem Pharmacol. 2002;64(5–6):963–70. doi: 10.1016/s0006-2952(02)01161-9. [DOI] [PubMed] [Google Scholar]
- 37.Chen JS, Hsu YM, Chen CC, Chen LL, Lee CC, Huang TS. Secreted heat shock protein 90alpha induces colorectal cancer cell invasion through CD91/LRP-1 and NF-kappaB-mediated integrin alphaV expression. J Biol Chem. 2010;285(33):25458–66. doi: 10.1074/jbc.M110.139345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marks-Konczalik J, Chu SC, Moss J. Cytokine-mediated transcriptional induction of the human inducible nitric oxide synthase gene requires both activator protein 1 and nuclear factor kappaB-binding sites. J Biol Chem. 1998;273(35):22201–8. doi: 10.1074/jbc.273.35.22201. [DOI] [PubMed] [Google Scholar]
- 39.Eum HA, Park SW, Lee SM. Role of nitric oxide in the expression of hepatic vascular stress genes in response to sepsis. Nitric Oxide. 2007;17(3–4):126–33. doi: 10.1016/j.niox.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Florczyk UM, Jozkowicz A, Dulak J. Biliverdin reductase: new features of an old enzyme and its potential therapeutic significance. Pharmacol Rep. 2008;60(1):38–48. [PMC free article] [PubMed] [Google Scholar]
- 41.Pachori AS, Smith A, McDonald P, Zhang L, Dzau VJ, Melo LG. Heme-oxygenase-1-induced protection against hypoxia/reoxygenation is dependent on biliverdin reductase and its interaction with PI3K/Akt path-way. J Mol Cell Cardiol. 2007;43(5):580–92. doi: 10.1016/j.yjmcc.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kobayashi T, Sugawara Y, Ohkubo T, Imamura H, Makuuchi M. Effects of amri-none on hepatic ischemia-reperfusion injury in rats. J Hepatol. 2002;37(1):31–8. doi: 10.1016/s0168-8278(02)00084-3. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi T, Shimizu H, Morimatsu H, Maeshima K, Inoue K, Akagi R, et al. Heme oxygenase-1 is an essential cytoprotective component in oxidative tissue Injury induced by hemorrhagic shock. J Clin Biochem Nutr. 2009;44(1):28–40. doi: 10.3164/jcbn.08-210-HO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pachori AS, Melo LG, Hart ML, Noiseux N, Zhang L, Morello F, et al. Hypoxia-regulated therapeutic gene as a preemptive treatment strategy against ischemia/reperfusion tissue injury. Proc Natl Acad Sci USA. 2004;101(33):12282–7. doi: 10.1073/pnas.0404616101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harstad EB, Klaassen CD. iNOS-null mice are not resistant to cadmium chloride-induced hepatotoxicity. Toxicology. 2002;175(1–3):83–90. doi: 10.1016/s0300-483x(02)00068-9. [DOI] [PubMed] [Google Scholar]
- 46.Kan WH, Hsu JT, Schwacha MG, Choudhry MA, Raju R, Bland KI, et al. Selective inhibition of iNOS attenuates trauma-hemorrhage/resuscitation-induced hepatic injury. J Appl Physiol. 2008;105(4):1076–82. doi: 10.1152/japplphysiol.90495.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wunder C, Scott JR, Lush CW, Brock RW, Bihari A, Harris K, et al. Heme oxygenase modulates hepatic leukocyte sequestration via changes in sinusoidal tone in systemic inflammation in mice. Microvasc Res. 2004;68(1):20–9. doi: 10.1016/j.mvr.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 48.Lin HI, Wang D, Leu FJ, Chen CF, Chen HI. Ischemia and reperfusion of liver induces eNOS and iNOS expression: effects of a NO donor and NOS inhibitor. Chin J Physiol. 2004;47(3):121–7. [PubMed] [Google Scholar]
- 49.Pasupuleti N, Gangadhariah M, Padmanabha S, Santhoshkumar P, Nagaraj RH. The role of the cysteine residue in the chaperone and anti-apoptotic functions of human Hsp27. J Cell Biochem. 2010;110(2):408–19. doi: 10.1002/jcb.22552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ishikawa H, Jin MB, Ogata T, Taniguchi M, Suzuki T, Shimamura T, et al. Role of cyclic nucleotides in ischemia and reperfusion injury of canine livers. Transplantation. 2002;73(7):1041–8. doi: 10.1097/00007890-200204150-00005. [DOI] [PubMed] [Google Scholar]