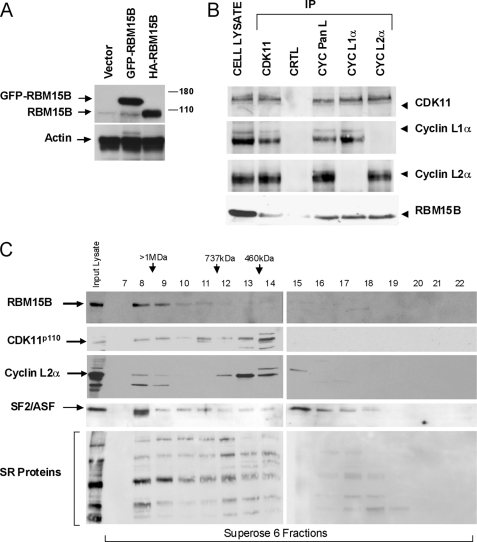
FIGURE 1.
CDK11p110-cyclin L and RBM15B co-immunoprecipitate and are present in large nuclear macromolecular complexes. A, characterization of RBM15B antibody. An anti-RBM15B-specific rabbit polyclonal antibody was raised against the GST-N-term domain of RBM15B (amino acids 1–154; supplemental Data S8) and affinity-purified (supplemental Data S2, B–D). Immunoblots were performed with this affinity-purified antibody using cell lysates from HEK293T cells transfected with empty vector or GFP- or HA-tagged RBM15B expression vectors. Actin was used as loading control. B, co-immunoprecipitation of CDK11p110 and cyclin L with RBM15B. Endogenous CDK11p110 and cyclin L proteins were immunoprecipitated from HEK293T cell lysates with anti-CDK11 (P1C), -cyclin (CYC) pan-L, -cyclin L1α, and -cyclin L2α antibodies. Purified rabbit IgG was used as a negative control (CTRL). Immunoprecipitates were analyzed by immunoblotting using CDK11 (P1C), cyclin L1α, cyclin L2α, and RBM15B antibodies. C, co-elution of CDK11p110, cyclin L2α, SR proteins, and RBM15B following size exclusion chromatography. Extracts from HeLa nuclei (NE; 1 mg) were fractionated using a Superose 6 column and analyzed by immunoblotting using the CDK11 (P1C), cyclin L2α, SF2/ASF, anti-SR protein (1H4 mAb), and RBM15B antibodies. The anti-SR antibody recognizes SRp75, SRp55, SRp40, SRp30a/b, and SRp20 proteins. Input lanes contain 20 μg of HeLa NE, whereas other lanes contain 40 μl from each 1-ml fraction.