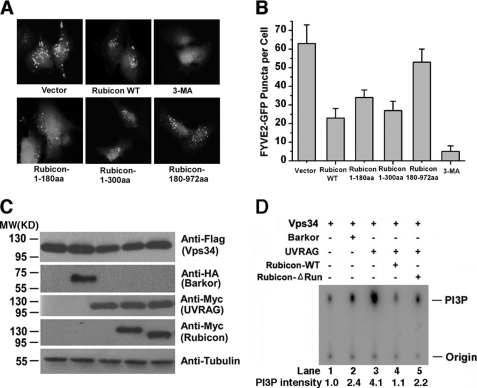
FIGURE 4.
The RUN domain of Rubicon contributes to efficient inhibition of hVps34 lipid kinase activity. A, vector alone, the N-terminal region (aa 1–300), and the C-terminal region (aa 181–972) of Rubicon were coexpressed with GFP-2×FYVE in HEK293T cells. GFP-2×FYVE puncta were observed under a fluorescence microscope. 3-MA, 3-methyladenine. B, quantitative analysis (summarized from 100 cells) of GFP-2×FYVE puncta in the cells described in A. C, FLAG-tagged hVps34 was coexpressed in HEK293T cells with one of the following Myc-tagged proteins: Beclin 1, Barkor/Atg14(L), UVRAG, Rubicon, or Rubicon-ΔRUN. D, cell lysates from the cells described in C were immunoprecipitated with M2 beads and assayed for PI3K kinase activity in a TLC assay. The phosphorylation product [32P]PI(3)P was separated by TLC and further visualized using a Fuji phosphorimaging scanner.