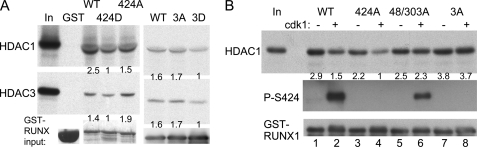
FIGURE 4.
Mutation of RUNX1 serine 424 to aspartic acid or phosphorylation by cdk1 reduces HDAC interaction using partially purified proteins. A, GST, GST-RUNX1 (WT), GST-RUNX1-S424D (424D), GST-RUNX1-S424A (424A), GST-RUNX1(tripleA) (3A), or GST-RUNX1(tripleD) (3D) proteins bound to glutathione-agarose beads were incubated with 35S-labeled HDAC1 or HDAC3 generated by in vitro transcription and translation, followed by washing, SDS-PAGE, and autoradiography. Equal volumes of input GST protein samples were also subjected to SDS-PAGE followed by Coomassie Blue dye staining (left panels) or RUNX1 Western blotting (right panels) to assess total protein expression. HDAC1 or HDAC3 band intensities relative to input GST proteins were quantified, as shown below each lane. B, GST-RUNX1, GST-RUNX1-S424A, GST-RUNX1-S48A/303A, or GST-RUNX1(tripleA) (3A) bound to agarose beads were incubated with or without cdk1/cyclin B in the presence of kinase buffer and ATP. The beads were then incubated with 35S-labeled HDAC1 followed by washing, SDS-PAGE, and autoradiography. Phosphorylation of Ser-424 was confirmed by Western blotting with anti-phospho-Ser-424 RUNX1 antiserum (middle panel), and GST-RUNX1 input was assessed by Western blot analysis of equal volumes of input samples using anti-RUNX1 antibody (bottom panel). HDAC1 band intensities relative to GST-RUNX1 were quantified, as shown below each lane of the top panel.