FIGURE 1.
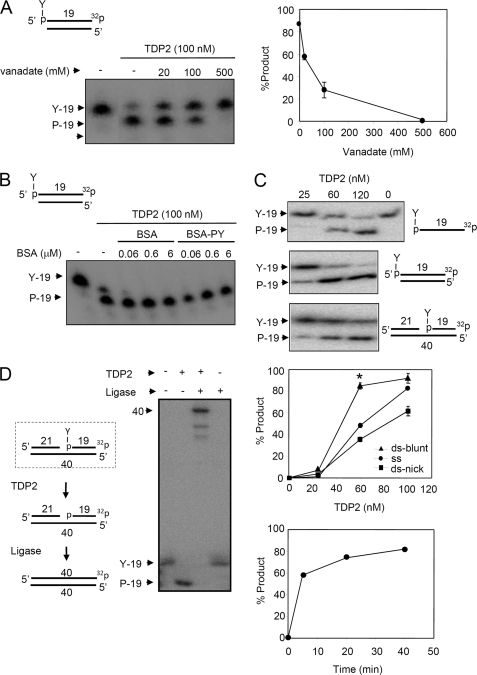
The 5′-TDP activity of His-TDP2 prefers DSBs and liberates ligatable 5′ termini from DNA 5′-phosphotyrosine termini. A, purified recombinant human His-TDP2 (100 nm) was incubated with 50 nm duplex substrate harboring a DSB with a 5′-phosphotyrosine (P-Y) terminus (top) in the absence or presence of the indicated concentrations of orthovanadate for 1 h at 37 °C and reaction products separated by denaturing PAGE and detected by autoradiography. Right, quantification of the fraction (%) of total labeled oligonucleotide converted to 5′-phosphate (19-mer) product is shown. Data are the average (± range) of two independent experiments. B, purified recombinant human His-TDP2 (100 nm) was incubated with duplex substrate as above in the absence or presence of the indicated concentrations of BSA or phosphotyrosine-BSA. C, DNA substrates with a 5′-phosphotyrosine terminus present in single-stranded DNA (top), in a blunt-ended DNA duplex (middle), or in a nicked duplex (bottom), were incubated with the indicated concentrations of His-TDP2 for 1 h at 37 °C and reaction products analyzed as above. The middle graph is the quantification (% of total labeled oligonucleotide converted to 5′-P 19-mer product) of the above experiment, from three independent experiments mean (±S.E.). Asterisk denotes statistically significant difference (p < 0.05; t test) between the indicated blunt-ended DNA duplex (ds-blunt) and single-stranded DNA (ss) data points. The bottom graph is a time course of TDP2 activity (120 nm) on the blunt-ended DNA duplex (50 nm) from a single experiment. D, nicked DNA duplex harboring a 5′-phosphotyrosine terminus (dotted box, left) was incubated with (+) or without (−) 200 nm human His-TDP2 and 50 nm Lig3α and reaction products analyzed as described above. The predicted reaction steps are depicted (left).