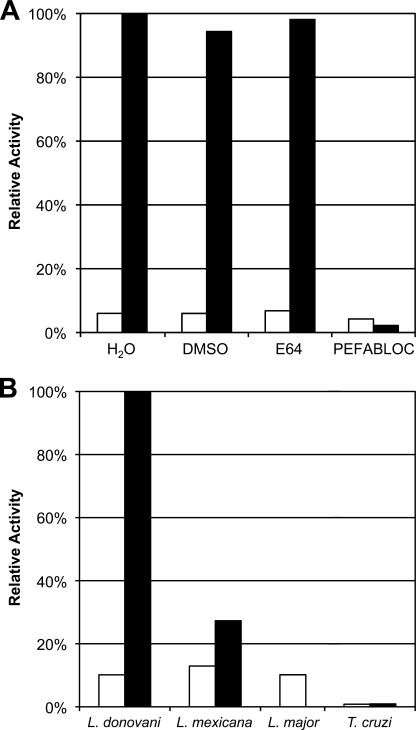
FIGURE 1.
Identification of high serine protease activity in Leishmania lysate. A, the soluble fraction of L. donovani lysate was tested for protease activity using a Z-PR-AMC substrate. Activity assays were performed at pH 5.5 (white bar) and at 8.0 (black bar), pH levels that are generally preferred by cysteine and serine proteases, respectively. The activity observed was tested for sensitivity to the cysteine protease inhibitor E-64 (10 μm) and the serine protease inhibitor PEFABLOC (2 mm). The DMSO concentration was 1%. Activities were normalized to the protease activity observed at the indicated pH without inhibitor. B, OPB activity was compared between three species of Leishmania and T. cruzi. Insect vector stages of the parasites (Leishmania promastigotes and T. cruzi epimastigotes, white bar) were compared with the vertebrate host stages (amastigotes, black bar) for L. donovani, L. mexicana, and T. cruzi. Equivalent amounts of protein from lysed parasites were measured for OPB activity at pH 8.0 using the Z-PR-AMC substrate.