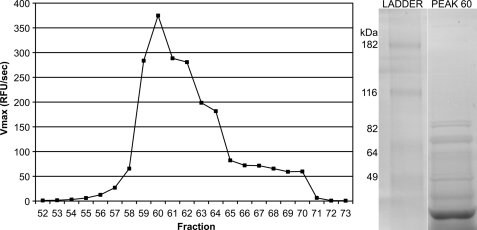
FIGURE 2.
Purification scheme and identification of OPB from L. donovani lysate. The soluble fraction of L. donovani promastigote lysate was fractionated by Q-Sepharose ion exchange chromatography. OPB-containing fractions were identified by testing for cleavage of Z-PR-AMC at pH 8.0. Activity was plotted against fraction number, and a single peak of activity was observed. The maximally active fraction, fraction 60, was resolved on an SDS-polyacrylamide gel. Major bands were excised from the gel, and the proteins were identified by tandem mass spectrometry. The protein contained in the excised gel from 75 to 90 kDa was identified as OPB. OPB has a predicted molecular mass of 83.1 kDa.