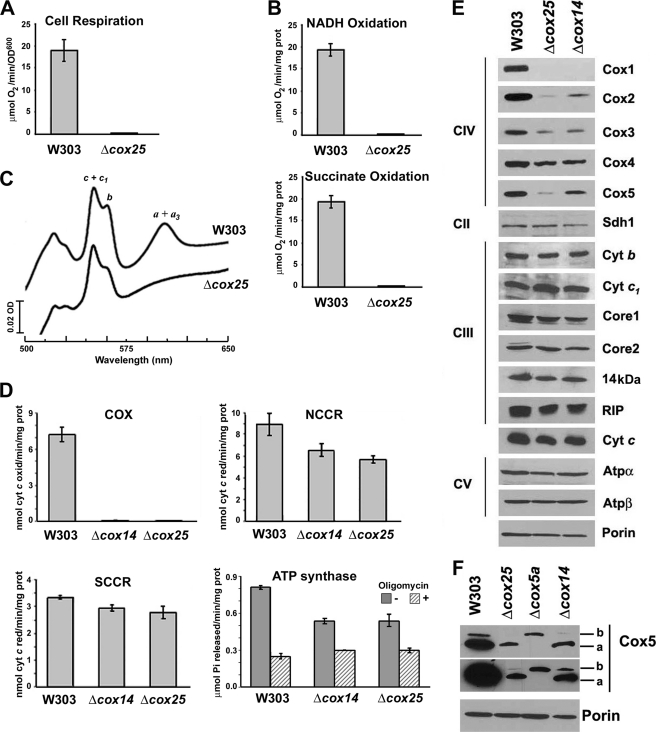
FIGURE 2.
Biochemical properties of Δcox25 cells. Respiratory assays A, KCN-sensitive endogenous cell respiration measured polarographically in the presence of galactose. B, rate of NADH and succinate oxidation in isolated mitochondria. C, total mitochondrial cytochrome spectra. Mitochondria from the wild-type strain W303 and the null mutant Δcox25 cells were extracted at a protein concentration of 5 mg/ml with potassium deoxycholate under conditions that quantitatively solubilize all of the cytochromes (36). Difference spectra of the reduced (sodium dithionite) versus oxidized (potassium ferricyanide) extracts were recorded at room temperature. The absorption bands corresponding to cytochromes a and a3 have maxima at 603 nm (a and a3); the maxima for cytochrome b (b) and for cytochrome c and c1 (c and c1) are 560 and 550 nm, respectively. D, mitochondrial respiratory chain enzyme spectrophotometric measurements in isolated mitochondria. COX, NADH cytochrome c reductase (NCCR), succinate cytochrome c reductase (SCCR), and ATP synthase activities were measured as described under “Experimental Procedures.” E, steady state concentrations of mitochondrial respiratory chain complexes IV (COX), II (Sdh), and III (bc1 complex) and ATP synthase subunits estimated by Western blot analyses of proteins separated in a 12% Tris-glycine SDS-PAGE. F, steady state concentrations of COX subunit 5 isoforms estimated by Western blot analyses of proteins separated in a 16.5% Tris-Tricine SDS-PAGE. Two expositions of the film are shown. In E and F, an antibody against porin was used to normalize the signals for protein loading.