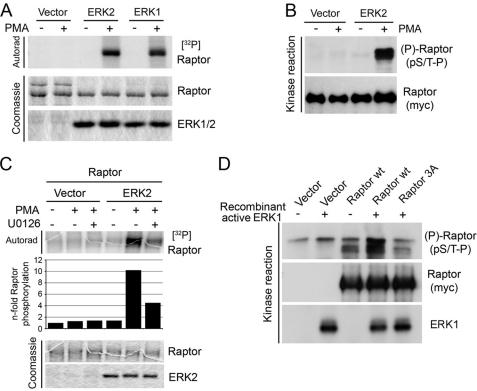
FIGURE 2.
ERK1/2 phosphorylate Raptor in vitro. A, immunoprecipitated HA-tagged WT ERK1 or HA6-tagged ERK2 from serum-starved or PMA-stimulated cells lysed in a Nonidet P-40 containing buffer was incubated with immunopurified Raptor in a kinase reaction with [γ-32P]ATP. The resulting samples were subjected to SDS-PAGE, and the dried Coomassie Blue-stained gel was autoradiographed. B, samples were treated as in A, but kinase reactions were performed without radioactivity. The resulting samples were immunoblotted for Raptor phosphorylation on pS/T-P consensus sites. C, as in A, but ERK2 was immunoprecipitated from cells treated with 50 ng/ml PMA in the presence or absence of 10 μm U0126. The levels of 32P incorporation into Raptor were quantified and are shown below the autoradiogram. D, recombinant activated ERK1 was incubated with immunopurified WT or S3A Raptor in a kinase reaction, and the resulting samples were immunoblotted for Raptor phosphorylation on pS/T-P consensus sites.