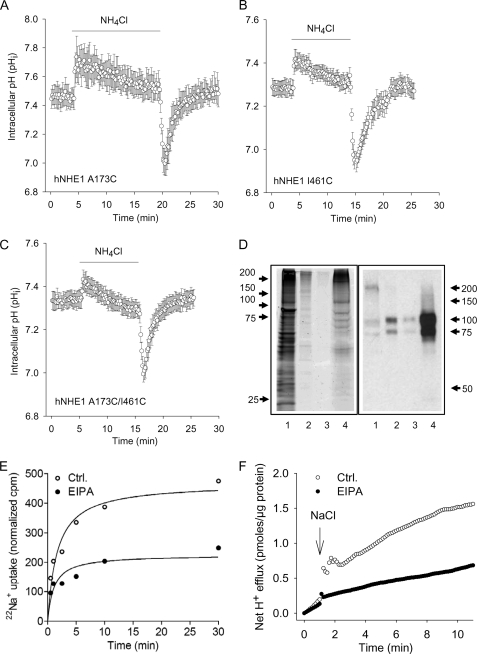
FIGURE 5.
Functional evaluation of the NHE1 constructs in AP1 cells and liposomes. A–C, regulation of pHi after an acid load in AP1 cells expressing the A173C (A), I461C (B), or A173C/I461C hNHE1 (C). AP1 cells were loaded with 2′,7′-bis-(2-carboxyethyl)-5,6-carboxyfluorescein and mounted on a Zeiss Axiovert S100 microscope. The cells were perfused with nominally HCO3−-free HEPES-buffered isotonic Ringer solution that, where indicated by the bar, additionally contained 10 mm NH4Cl. Calibration to pHi was carried out as previously described (12). The data shown are representative of six or seven independent experiments/condition. The rates of pHi recovery, obtained at similar starting pHi values but not normalized to expression levels, were 0.13 ± 0.010 (A173C, n = 7), 0.10 ± 0.0039 (I461C, n = 6), and 0.17 ± 0.0037 (A173C/I461C, n = 6). D, Coomassie Fluor Orange staining and Western blot of the A173C/I461C hNHE1 mutant after purification. Purification, staining, and immunoblotting were carried out as described under “Experimental Procedures.” Coomassie Fluor Orange staining is shown in the left panel, and Western blotting for NHE1 is shown in the right panel. Lane 1, the solubilized membrane fraction before transfer to the Ni2+ column; lane 2, eluate from Ni+ column with Ni+ elution buffer (containing 300 mm imidazole); lane 3, eluate from the CaM column with CaM elution buffer; lane 4, purified A173C/I461C hNHE1 sample obtained from the concentration of the sample shown in lane 3. E and F, 22Na+ uptake and H+ flux by hNHE1 reconstituted in liposomes. E, 22Na+ uptake was measured over time in hNHE1 liposome suspensions. The assays were performed in high osmolarity buffered sucrose solution and initiated by the addition of tracer 22Na+ containing solution. F, hNHE1 liposomes were suspended in a poorly buffered KCl medium, monitored with a pH microelectrode in the external bath solution, and expressed as H+ flux. The H+ flux reaction was initiated by adding 10 μl of 4 m NaCl solution at the time indicated. Where indicated, the liposomes were suspended in EVM in the presence of 50 μm EIPA. The difference caused by EIPA represents the NHE1-specific flux. Ctrl., control.