Figure 5. Perivitelline injection of heparin inhibits Dpp signaling and disrupts embryonic dorsal/ventral patterning.
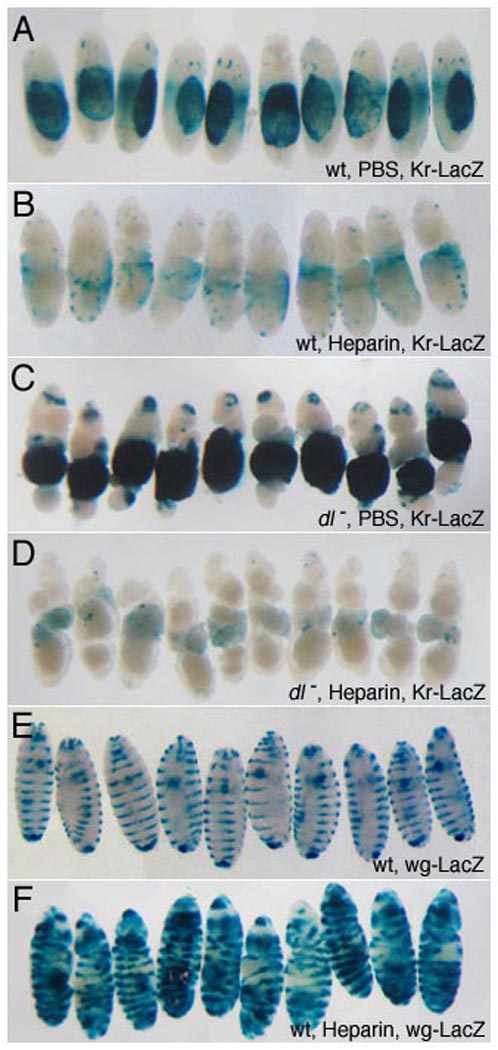
Heparin/PBS solution was injected into the perivitelline space of 1–2 hour embryos to achieve the specified final concentrations (see Methods). Embryos were allowed to develop for 21 hours before fixation and staining. The dorsal-most embryonic tissue, the amnioserosa, is marked by expression of a Kr-lacZ transgene (A–D) that provides a readout of alterations in D/V patterning. Representative embryos are shown; the total sample size is indicated in parenthesis for each genotype. Injection of heparin at 1.5 µg/ml leads to reduction in Kr-LacZ expression (A, n=136, B, n=114) and morphological defects typical of loss of dorsal cell fates, such as expansion of the cephalic furrow. In dl embryos, ubiquitous dpp expression results in ventral expansion of reporter expression (C, n=40). Injected heparin (10.5 µg/ml) inhibits reporter expression in embryos lacking dl activity, demonstrating that heparin directly interferes with BMP signaling (D, n=37). In contrast, embryonic morphology along the A/P axis and segmental expression of a wg-lacZ, reporter (E), are unaffected by heparin (1.5 µg/ml), (F, n=121). This indicates that A/P patterning and Wg/Hh activity are not compromised at heparin levels that disrupt BMP signaling, providing a control for specificity. Expansion of wg-lacZ stripes laterally so that they encircle the embryo reflects cell fate changes resulting from ventralization.
