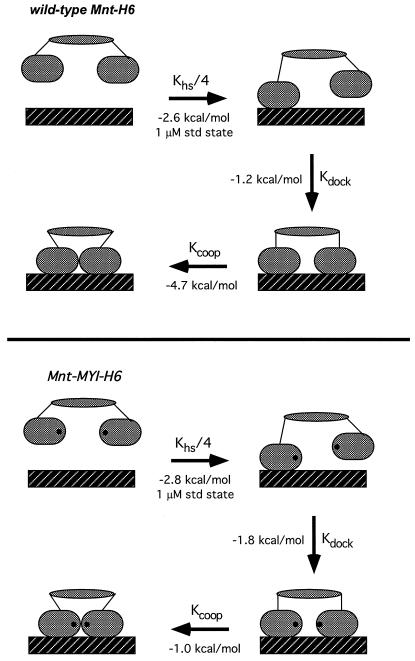
Figure 4.
Models for operator binding of the wild-type and MYI Mnt tetramers divided into discrete steps: (i) bimolecular binding of one dimeric DNA-binding domain to a single operator half-site; (ii) intramolecular docking of the second dimeric DNA-binding domain to the adjacent operator half site; and (iii) formation of cooperative contacts between DNA-binding domains. Equilibrium constants were calculated from the data in Fig. 3, as described in the text. ΔG values are calculated for a standard state of 1 μM for the bimolecular reaction and are within error for steps (i), and (ii) for the wild-type and mutant proteins. ΔGcoop for step (iii) is dramatically reduced for the MYI mutant.