Abstract
The universal temperature-dependence model (UTD) of the metabolic theory of ecology (MTE) proposes that temperature controls mass-scaled, whole-animal resting metabolic rate according to the first principles of physics (Boltzmann kinetics). Controversy surrounds the model's implication of a mechanistic basis for metabolism that excludes the effects of adaptive regulation, and it is unclear how this would apply to organisms that live in fringe environments and typically show considerable metabolic adaptation. We explored thermal scaling of metabolism in a rocky-shore eulittoral-fringe snail (Echinolittorina malaccana) that experiences constrained energy gain and fluctuating high temperatures (between 25°C and approximately 50°C) during prolonged emersion (weeks). In contrast to the prediction of the UTD model, metabolic rate was often negatively related to temperature over a benign range (30–40°C), the relationship depending on (i) the temperature range, (ii) the degree of metabolic depression (related to the quiescent period), and (iii) whether snails were isolated within their shells. Apparent activation energies (E) varied between 0.05 and −0.43 eV, deviating excessively from the UTD's predicted range of between 0.6 and 0.7 eV. The lowering of metabolism when heated should improve energy conservation in a high-temperature environment and challenges both the theory's generality and its mechanistic basis.
Keywords: gastropod, hypometabolism, metabolic rate depression, Littorinidae, thermal scaling
1. Introduction
The metabolic theory of ecology (MTE) proposes universal relationships among body mass, temperature and metabolic rate in living organisms [1–4]. Although the thermal pillar of the theory, the universal temperature-dependence model (UTD), is widely supported among studied species (including unicellular organisms, plants and ectothermic and endothermic animals), controversy surrounds whether the model represents a mechanistic basis for metabolic rate [5–7]. Metabolic rate is predicted to vary with temperature (T) according to the Boltzmann–Arrhenius factor (e−E/kT), where E is the activation energy of the biochemical reaction and k is Boltzmann's constant [1]. Opponents argue that this does not account for metabolic regulatory and feedback processes moulded by evolutionary trade-offs and temperature adaptation [5–9]. Much of the controversy concerns adaptive metabolic regulation in the form of thermal acclimation (metabolic compensation over weeks of temperature change; [5]), which proponents believe is explained by the normalization constant (b0 or intercept) of the general equation (B = b0M3/4e−E/kT; [3]). Departures from the model's prediction of acute metabolic responses to temperature (the slope) have been shown for insects and arachnids [10,11], but whether the UTD model has a purely mechanistic basis remains incompletely explored. For instance, tests of the model give little consideration to organisms (such as ectothermic animals) from common but ‘stressful’ thermal environments, which are likely to show greater metabolic adaptation than most. In other words, support for the theory could reflect the study of animals that show similar kinds of thermal adaptation, while animals from fringe environments that could deviate from general patterns are excluded or considered as outliers. Furthermore, where deviations from the model have been shown, this is assumed to reflect metabolic adaptation, but there has been little consideration of the environmental drivers of such adaptation (see [5,10,11]). Here, we explore the generality and the mechanistic basis of the UTD model through an investigation of an organism from a ‘stressful’ environment, a rocky-shore eulittoral-fringe snail (Echinolittorina malaccana). This habitat is found worldwide and in the tropics this snail faces the theoretical energetic problem of living under costly fluctuating high temperatures while experiencing a lifelong constraint on feeding and energy gain [12].
Most animals conform to classical evolutionary ecological theory based on selection for maximization of energy gain during periods of activity (see [2,13,14]), but there are situations in which minimizing energy loss becomes more important than maximizing its gain. For instance, animals that spend the greater part of their lifetime in a resting state and consequently experience constrained opportunities for energy gain must control energy balance by reducing costs. In aestivating invertebrates this is achieved through metabolic rate depression (see [15,16]). Although classical models place little value on the contribution of rest to improving fitness, metabolically depressed states of aestivating animals are nevertheless relevant to the MTE [1,2]. While there are no data showing that metabolically depressed states comply with the predictions of the UTD model, this is generally implied. For example, Guppy & Withers [15] used a Q10 of 2.5 to standardize the effect of temperature on depressed metabolism in comparisons between species. Thermal scaling of metabolism based on the UTD's prediction may be appropriate to animals that aestivate in stable environments, but this would seem maladaptive to shoreline marine snails aestivating in thermally variable environments, as it reduces the energetic benefit derived from the depressed metabolic state when temperatures rise. Temperature-independent metabolism and metabolic rate depression are well-known energy-conserving features of marine snails [17–21], but the environmental drivers of hypometabolism are usually taken to include dehydration, anoxia and hypersalinity [15,22–24]. Little is known about heat induction of hypometabolism and we know of no investigations of the thermal scaling of depressed metabolism in these or any other animal.
Echinolittorina snails live at the top of the shore and typically spend more than 90 per cent of their lifetime exposed to air, in a state of rest [25]. Adaptations for prolonged aerial exposure include mechanisms to limit desiccation and enhance aerobic efficiency [26,27]. These snails can tolerate extraordinarily high-temperature exposure (LT50 for E. malaccana = 56.5°C; [12]), but cannot be regarded as hyperthermaphiles, which fall outside the context of the MTE [1]. They avoid using energetically expensive anaerobic metabolism during aerial exposure (which contributes less than 2% of total ATP turnover; [21]), and conserve energy by depressing aerobic metabolism, potentially to below 20 per cent of the standard metabolic rate [21]. Our objective was to assess compliance of depressed metabolism in Echinolittorina snails to the UTD model. This required the initial determination of the maximum level to which metabolism is depressed, and of the behavioural and environmental drivers of metabolic depression. We compared the metabolism of snails that were allowed to isolate and withdraw into their shells for different periods of time, with that of resting snails that were prevented from isolating by being kept in humid air. The study concerned thermal scaling of steady-state, whole-animal aerobic depressed metabolic rates for different periods of quiescence and not the induction of metabolic depression itself. From the complimentary measurement of oxygen consumption and heart performance, we compared metabolic rates at two benign temperatures that fall within the thermal framework of the UTD model (30°C and 40°C; [1]). The respiratory data provided quantitative information on metabolic depression, while heart performance allowed assessment of metabolic rate variation under acutely changing temperature conditions (heating and cooling).
2. Material and methods
(a). Species and habitat
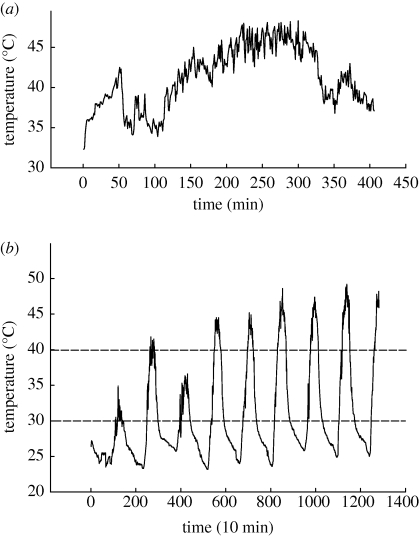
Specimens of E. malaccana (formerly Echinolittorina trochoides and Nodilittorina trochoides, [28]) were collected from artificial seawalls at Tungku or Jerudong, Brunei Darussalam (4°32′ N; 114°43′ E) between January 2008 and April 2009. They occur mainly in the eulittoral fringe zone from 2 m Chart Datum to around 5 m above the mean high water level (2.5 m CD). Similar sized individuals (shell length = 7–10 mm) that were feeding at the lower vertical distribution of these snails were returned to the laboratory. They were washed in filtered sea water (28 psu), allowed to emerge from their shells and to reattach to lidded plastic Petri dishes. Other than being exposed to laboratory temperatures (22°C) during manipulation, snails experienced only benign pre-experimental and experimental temperatures ranging between 30°C and 40°C. These temperatures are well below the Arrhenius breakpoint temperature (ABT = 53.9°C) for heart rate (HR), which marks the onset of thermal stress, and the upper lethal temperature [8,12]. The critical temperatures of closely related littorinid snails from temperate regions however fall within the model's thermal range of 0–40°C [29]. Preliminary experiments showed no mortality of Echinolittorina snails (N = 40) kept at a constant 40°C in dry air for 4 days. Continuous recordings from within a silicone-filled shell glued to a rock exposed to direct sunlight near to where snails were collected, showed that aerial ‘body’ temperatures rose above 40°C for more than 4 h on 5 days (figure 1). Daily minima were around 24°C and daily maxima between 35 and 49°C, depending on the sunlight conditions. Maxima of approximately 52°C have been recorded elsewhere [12]. Mean and maximum rate of temperature change recorded on 1 day at 1 min intervals were, respectively, 0.962 and 4.3°C min−1 (figure 1a). Although the snails are known to thermoregulate behaviourally by seeking shade, lifting their shells off the rock surface and orienting to the sun, this was not observed in any collected individuals.
Figure 1.
(a) Field temperatures recorded every 1 min between 9.00 and 15.00, from within a silicone-filled shell glued to a rock directly exposed to sunlight. A fine K-type thermocouple (Cromega) and a Fluke 54 Series II recording digital thermometer were used and data were downloaded to Fluke Viewforms and MS Excel files. (b) Temperature recordings (as above) but logged every 10 min for 9 days. Dashed horizontal lines indicate the stable temperatures used in laboratory experiments.
(b). Oxygen consumption experiments
Isolation behaviour in littorinid snails during aerial exposure is naturally induced by drying conditions. The metabolic activity of air-exposed snails was assessed under humid and dry conditions for different periods of emersion. Experiments were performed on field fresh (recently fed) snails collected within 2 h of the experiment and snails that were kept in an incubator (Memmert UFE 500, Schwabach, Germany) at 30°C for 2–3 days (2 days), 7–10 days (7 days) or 42–45 days (42 days). For the 2 days and 7 days treatments, the Petri dishes holding the snails either contained a small volume of water to create humid conditions or were kept dry. Only the dry condition was used for the 42 days aestivation treatment. To test whether the effect on oxygen consumption of the dry treatment related to water loss from snails, additional groups of 7 days dry snails were prepared, but in this case the snails were placed in sea water (28 psu) and allowed to emerge from their shells before undertaking respirometry (7 days wet snails). To induce isolation rapidly in the dry treatment or to dry shells before dry air respirometry, snails were exposed to fan blown air inside the incubator for 1–2 h (30°C). While isolation behaviour in dry air was characterized by withdrawal into the shell and closure of the operculum, under humid conditions the snails remained attached with their foot expanded. Even under drying conditions the snails resist water loss; mean per cent weight loss per hour (±1 s.e.m.) determined for the dry snails in the incubator over 32 days (30°C) was 0.01 ± 6.7 × 10−3 (N = 10).
Respirometry was performed in either humid (presumed 100% water saturation) or dry air (presumed 0% saturation), controlled, respectively, by liberally wetted or activated silica gel comprising around 40 per cent of the respirometer's volume. Humid respirometry always followed a humid pre-treatment or was used for field fresh snails while dry respirometery followed a dry pre-treatment or was used for 7 days wet snails. Eight replicates were used for all treatments and each replicate comprised a group of between 10 and 14 snails (0.220–0.310 g wet weight sample−1, see [21,30]). During any experiment the same group of snails was first exposed to 30°C then to 40°C. Respirometers comprised wide-neck McCarthy bottles (7 ml) with rubber-washered screw lids fitted with fine electrodes (MI-730 Micro-Oxygen Electrode, Microelectrodes, Inc. USA). The electrodes were coupled to adapters (MI O2-ADPT, Microelectrodes, Inc. USA) and a data acquisition system (ADInstruments, PowerLab/4SP, Castle Hill, Australia). Snails in respirometers were separated by nylon mesh from the silica gel in the lower part of the bottle and the oxygen electrode in the upper part. Electrodes were periodically calibrated in N2 and humid air according to the manufacturer's recommendations, and the drift for blanks containing no snails was always less than 2 per cent over 12 h at 40°C.
At the start of an experiment, respirometers containing snails were bagged and placed in a Grant programmable water-bath (GP 200) set to 30°C. To prevent condensation on the electrode during initial temperature elevation in the high humidity condition, silica gel granules were packed around it on the upper mesh; these became deactivated within the first 30 min of an experiment. PO2 (% air saturation) was recorded at a rate of 40 Hz for more than 4 h, and the average for every 1 min was logged (ADInstruments PowerLab 4/SP, Chart v. 5). Once recordings had stabilized to ambient pressure at 30°C (around 10 min), the upper scale was set to 21 per cent oxygen (air saturation), and respirometer caps were tightened. At the end of a recording session, the caps were reopened and the temperature was abruptly raised to 40°C (over 2 min). The entire procedure was repeated at this temperature and recordings were made over 12 h. After determining oxygen consumption at both temperatures, the air volume within the respirometers, excluding that occupied by the snails and other components, was determined by weighing the water that filled the free space (around 4.5 ml). Snails were then transferred to Petri dishes and flushed with aerated, filtered sea water (28 psu). All snails emerged from their shells and attached their foot within 30 min; mortality was only observed for four of more than 500 snails used in all experiments. Wet tissue weight was determined for each snail group, after dissolving shells in 7 per cent nitric acid for 20 min, then vigorously washing in distilled water, and blotting dry (after McMahon et al. [30]). Dry tissue weight was then determined after oven drying at 60°C for 3 days. Change in PO2 (measured as % O2) was converted to micromoles of O2 using the ideal gas law equation and accounting for the water vapour pressure in the case of the humid treatment; final data are given in micromoles of O2 per gram of wet weight−1(wet weight = 0.014 + 2.94 dry weight, r2 = 0.9376, p < 0.001, N = 21). Apparent activation energy for oxygen uptake rate between 30 and 40°C was calculated from the formula: E = [(ln k1 − ln k2).R]/ [1/T2 − 1/T1], where R is the universal gas constant, and k1 and k2 are the rates at temperatures T1 and T2, respectively. To convert kilojoules per mole to electronvolts, values were divided by 96.48.
(c). Heart performance experiments
Heart performance was measured to assess the effect of dynamic temperature change (heating and cooling) on individual snail metabolism. Plethysmographic traces were obtained using optoelectronic (infrared) sensors (Vishay Semiconductors, CNY70) adhered to the shells of snails near the mantle cavity (with Blu-Tac, Bostick). Snails (N = 10) that were field fresh or had been kept for between 1 and 16 days in dry conditions (at 30°C) were exposed to cycling air temperatures and ambient humidity in a programmable Peltier-cooled Memmert (IPP 400) incubator. Each cycle comprised five ramps: R1, 26–30°C over 30 min; R2, stable 30°C for 120 min; R3, elevating 30–40°C over 120 min; R4, stable 40°C for 240 min and R5, decreasing 40–30°C over 120 min. The programme entry caused a rapid dip in temperature to 25°C before the stable 30°C period. Signals from the sensors were amplified, filtered (using a custom-built pre-amplifier) and digitally logged using an ADInstruments PowerLab 4/SP (see above). Sampling rate was set at 40 Hz and the amplitude varied between 20 and 800 mV, depending on sensor placement and pre-condition of the snail. A smoothed cardiogram trace (triangular Bartlett smoothing) was derived on a separate channel. From the plethysmographic recordings, HR, stroke volume and cardiac output (the product of HR and stroke volume) were determined for 1 min intervals using Chart v. 5. Stroke volume (based on average cyclical height for each 1 min interval) was either coupled or uncoupled with HR, but varied by a much smaller factor than HR, resulting in cardiac output approximating the HR patterning over the temperature range used (see the electronic supplementary material, figure S1). The temperature within a silicone-filled shell held in the incubator during experiments was recorded every 1 min on a separate channel of the PowerLab system, using an ADInstruments MLT 1402 T-type ultra-fast thermocouple and a ML 312 T-type pod. Emergence of snails placed in water-containing vials (at 30°C for 24 h) after each experiment confirmed their good health.
(d). Analysis and statistics
In the oxygen consumption experiments, field fresh and 2 days humid snails were used to determine responses to an abrupt temperature change. Respiratory rates at 30°C and 40°C (repeated measures) of eight replicates for 1, 2 and 3 h were compared using one-way repeated measures ANOVA (RMANOVA, [31]). Rate was determined by averaging that measured every 1 min for 30–60 min (1 h), 61–120 min (2 h) and 121–180 min (3 h; see figure 3). RMANOVA was further used to compare steady-state respiratory rates following longer experimental times (averaged 1 min recordings between 4–8 h) for three other pre-treatments (7 days humid, 7 days dry and 2 days dry). Data were only available for the 40°C experimental temperature (4–8 h) in the case of 42 days dry and 7 days wet snails, and comparisons at the same temperature with 7 days humid, 7 days dry and 2 days dry snails were based on independent t-tests. Statistica v. 6.1 (Statsoft) was used for the statistical analyses and figures were drawn using Sigma Plot v. 8 (SPSS Inc.).
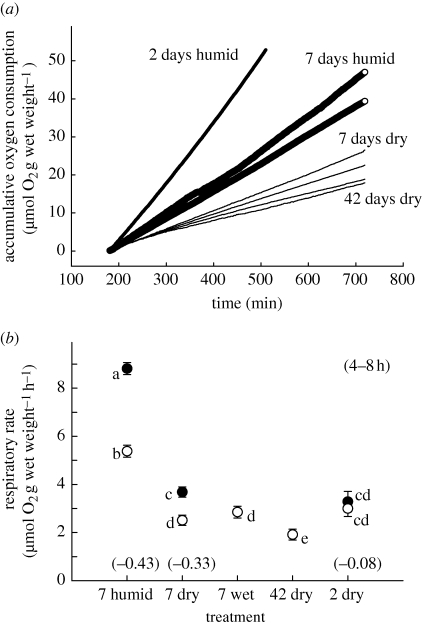
Figure 3.
(a) Comparison of accumulative oxygen consumption for seven representative groups of snails pretreated as indicated (increasing line thickness represents in order, 42 days dry (N = 2), 7 days dry (N = 2), 2 days humid (N = 1) and 7 days humid (N = 2)). Rate was measured every 1 min from zero, between 3 and 12 h at 40°C. This shows constancy of rate during this period after initial heat induced deceleration, and the effects of humidity and aestivation period on rate. (b) Effect of pretreatment on respiratory rate (least squared mean ± 1 s.e.m; N = 8 for all 7 days treatments, and N = 6 for the 42 days and 2 days treatments). Rates were calculated by averaging 1 min recordings for between 4–8 h exposure at 30 (closed) or 40°C (open symbols). In 7 wet (7 days dry snails wetted before respirometry) and 42 dry, rates were determined only at 40°C. Different letters indicate significantly different means (p < 0.01, using Tukey HSD test and RMANOVA, Cochran C more than 0.42 for 30 and 40°C; independent t-tests were used in comparisons with 7 wet and 42 dry). Apparent activation energies (eV) for rates between 30°C and 40°C are shown in parenthesis.
3. Results
(a). Oxygen consumption experiments
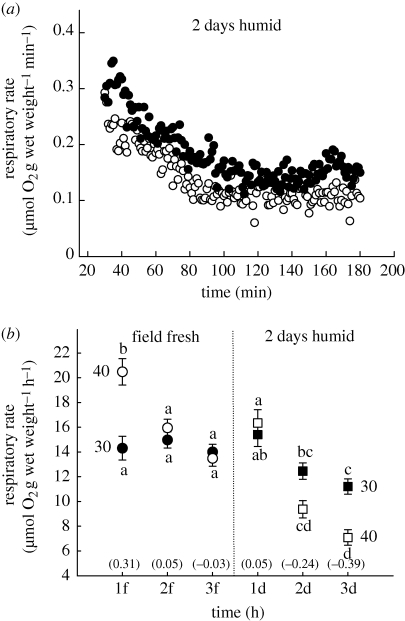
Resting aerial respiratory rate of Echinolittorina snails was highly variable and was regulated in relation to heating, aestivation state and humidity condition. Mean rate varied between 20.5 (for field fresh fed snails at 40°C) and 1.9 µmol O2 h−1 (for 42 days snails at 40°C, figures 2 and 3). Stable temperature exposure (at 30°C or 40°C) resulted in a nonlinear decrease in rate during the first 2–3 h (figure 2a), after which a constant rate was achieved and maintained for up to 12 h (figure 3a). For the most temperature-sensitive pre-treatments (field fresh and 2 days humid snails), the initial deceleration was greater at 40°C than at 30°C; hourly means were not significantly different for the first 3 h in field fresh snails at 30°C (figure 2b, p > 0.05). Stable respiratory rate (after 3 h) was generally more depressed in snails that had experienced 2 days humid air than in field fresh snails; mean rates (3 h, 40°C) were, respectively, 7.09 and 13.48 µmol O2 h−1 (figure 2b). Furthermore, stable respiratory rate (3 h) of 2 days humid snails was more depressed at 40°C than at 30°C; that for the latter was 11.21 µmol O2 h−1 (figure 2b).
Figure 2.
(a) Oxygen consumption rate plotted against time (for every 1 min between 30 and 180 min) for a single representative group of snails. Rate was determined in humid air, first at a constant 30°C (more than 3 h) and then at 40°C for the same snails kept in humid air for 2 days before the determination (30°C). Rate decelerated during the first 2 h at each temperature (following abrupt temperature elevation) and then became constant; in this case rate at 40°C (open circle) is suppressed below that at 30°C (filled circle). (b) Oxygen consumption rate (least-squared mean ± 1 s.e.m; N = 8 determinations) during the first 3 h at 30°C (closed symbols) and then during this time at 40°C (open symbols) for field fresh (circles) and 2 days humid snails (squares). Rate was determined by averaging 1 min recordings for 30–60 min (1f, 1d), 61–120 min (2f, 2d) and 121–180 min (3f, 3d). Different letters associated with symbols indicate significantly different means for within pretreatment comparisons (based on p < 0.01, Tukey HSD test and RMANOVA for all shown data, Cochran C more than 0.303 for 30°C and 40°C). Apparent activation energies (eV) for rates between 30°C and 40°C are shown in parenthesis.
Negative rate-temperature relationships of steady-state respiration were also observed for the other pre-treatments (7 days humid and 7 days dry; figure 3). Increasing the time of the humid pre-treatment to 7 days, reduced this rate by around 20 per cent of that of 2 days humid treatment (mean rates at 30°C and 40°C for 7 days humid snails were, respectively, 8.81 and 5.38 µmol O2 h−1; figure 3, p < 0.01). Steady-state respiration of snails in dry air was depressed below that of snails in humid air. There was, however, no significant difference in mean respiratory rate at 40°C between dry snails that had aestivated for 2 days (2.99 µmol O2 h−1) and those that had aestivated for 7 days (2.51 µmol O2 h−1; figure 3). Furthermore, wetting of 7 days dry snails did not affect respiratory rate (2.85 µmol O2 h−1), which was similar to that of 7 days dry snails without wetting (figure 3). However, a 42 days dry period significantly lowered the rate (1.91 µmol O2 h−1) below that of 7 days dry snails. While this represents the lowest respiratory rate determined for these snails, a single upper level comparator is less obvious; depression of aerial oxygen consumption during aestivation calculated from field fresh snails (3 h, 40°C) was 14.1 per cent, while a calculation based on 2 days humid snails (3 h, 40°C) was 26.9 per cent. Within 2 days, dry snails had depressed their metabolism by 22.1 per cent of that of field fresh snails. Apparent activation energies between 30°C and 40°C varied between 0.31 and −0.43 eV, depending on the time of exposure to a constant temperature, length of aestivation period, and conditions of humidity (figures 2 and 3). Considering that steady-state metabolism is only achieved around 2 h of stable temperature exposure, a more realistic range of activation energies is 0.05 to −0.43 eV (figure 2 and 3).
(b). Heart performance experiments
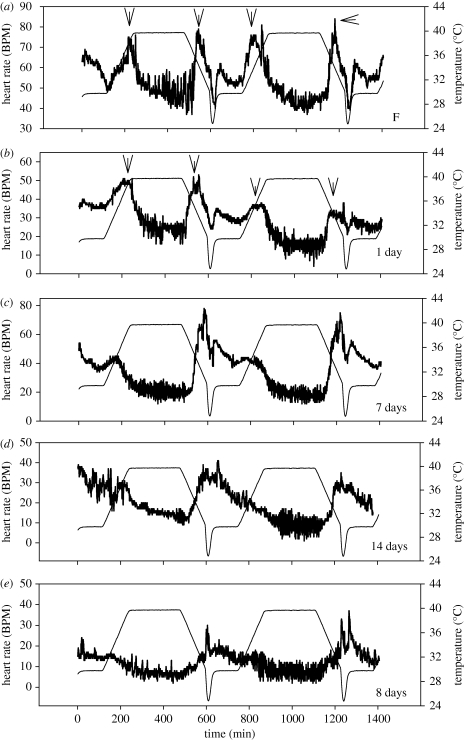
HR was similar to oxygen consumption rate in its response to temperature change and relation to aestivation state (figure 4). An acute temperature change (between 30°C and 40°C over 2 h) caused HR suppression within 2 h of exposure to the new stable temperature. Longer aestivation caused HR to become more suppressed and less temperature sensitive (figure 4). Suppression of HR at 40°C was significantly lower than that at 30°C (see the electronic supplementary material, table S1) and basal HR sometimes declined in successive cycles (figure 4b). In contrast to heating, which caused HR to fall, cooling caused elevation of suppressed rates; this could be associated with preparation for activity as evaporative cooling naturally occurs when snails are wetted and stimulated to feed. A novel response to heating or cooling was detected in some snails (freshly collected or in early aestivation); basal HRs that were coupled with temperature below around 35°C became uncoupled at higher temperatures when temperature was raised or lowered (see figure 4a,b). The scope of heart performance of Echinolittorina snails was remarkably broad; at 40°C this ranged from below 10 BPM in deeply aestivating individuals to 140 BPM in other individuals (D. J. Marshall 2009, unpublished data).
Figure 4.
Representative traces of heart rate patterning (thick lines) in relation to temperature variation (thin lines) for five individual snails aestivated (at 30°C) for different periods (shown at lower right of each panel; F-field fresh). Stable HR at 40°C is depressed below that at 30°C. This and sensitivity to temperature generally decrease with increasing aestivation period. Arrows in (a,b) indicate points where HR either becomes temperature-dependent or temperature-independent (mostly in the mid to upper 30°C) during heating or cooling.
4. Discussion
The UTD model proposes that, when scaled for body mass, the resting metabolism of organisms is controlled by temperature according to basic thermodynamic principles (the Boltzmann term, e−E/kT; [1–3]). Although most organisms examined so far show thermal scaling according to the model [1,3], recently deviations from the prediction of the model have been demonstrated [10,11] suggesting that the assumption of a mechanistic basis to the theory may be incorrect. Opponents of the theory have argued that Boltzmann kinetics inadequately explain adaptation of metabolism, but this has been met with the counterargument that thermal adaptation and acclimation are accounted for by the normalization constant (see §1), rather than specifically by the UTD term [3,5,6]. An alternative explanation for the fact that the slope of metabolic rate against temperature overwhelmingly conforms to the UTD's prediction could relate to the model's limited thermal framework (0–40°C). There is no clear basis for defining this thermal window and it could exclude exactly those organisms most likely to deviate from the model. The window covers the range of above zero temperatures encountered in many (perhaps most) environments, where organisms will require relatively little adaptation compared with species that commonly experience body temperatures above 40°C. Higher body temperatures are not confined to specialist hyperthermaphiles, and characterize many rocky shore invertebrates, some of which are adapted to conditions of direct solar radiative heating [12,32]. The relationship between metabolic rate and temperature in Echinolittorina snails was often the reverse of that described by Boltzmann kinetics. In other words, metabolic rate was lowered rather than raised over the range of higher temperatures commonly experienced during emersion (see figure 1). Activation energies of steady-state resting metabolism of E. malaccana fell between 0.05 and −0.43 eV, compared with ranges of 0.24–0.99 eV determined for insects, and 0.6–0.7 eV predicted by the UTD model [1,10]. For insects, the deviations from UTD predictions observed under moderate temperature conditions are explained by methodology [10], but for Echinolittorina the response of metabolic rate to temperature provides a compelling demonstration of the degree to which metabolic compensation can override Boltzmann kinetics in order to overcome the costs of living at high temperatures.
Most animals are incapable of lowering metabolism below some standard resting level, but the capacity for intrinsic metabolic depression is nonetheless widespread across the major phyla [14]. Metabolism is typically depressed to below 20 per cent of the standard resting level and involves structural and functional cellular modification, including a decrease in pH, the presence of latent mRNA, an altered phosphorylation state of proteins, maintenance of ion pumping and the down-regulation of protein synthesis [15]. Despite the general implication that depressed metabolic states conform to the UTD prediction, empirical studies in this respect are essentially lacking [1,15]. Animals with a relatively narrow aestivating thermal niche may comply with the UTD, but allowing temperature dependent metabolism would compromise the energetic benefits of aestivation in animals exposed to high and variable temperatures, such as Echinolittorina snails. We found that these snails exhibit a variety of previously unknown metabolic responses to temperature that depend on the degree of isolation from the environment and the period of quiescence. While the unstable metabolism occurring initially during acute temperature change in field fresh snails showed temperature scaling closest to the UTD's prediction (figure 2b), deviation from the prediction of steady-state metabolism generally increased with period of quiescence (figure 3b). Continuous HR recordings of recently rested snails suggest that at some stage thermal scaling of metabolism depends on the temperature range experienced; HR was dependent on temperature at temperatures below approximately 35°C and was thermally independent above this temperature. However, this pattern was lost and the thermal sensitivity of the HR largely decreased with longer quiescence. Although steady-state hypometabolism was achieved under both humid (in non-isolated snails) and dry aerial conditions (in isolated snails), deeper depression characterized the latter and consistently occurred in snails exposed to 40°C than those held at 30°C. The difference in metabolism between these temperatures (indicated by the slope of the rate-temperature relationship) was greater in non-isolated snails compared with snails isolated for similar quiescent periods. This relates to the relatively limited metabolic depression for all treatments in non-isolated snails at 30°C. Nonetheless, the fact that hypometabolism was induced in non-isolated snails indicates that it is important to saving energy during rest rather than being only associated with isolation and avoiding desiccation. The depth of metabolic depression was not related to internal water status, as isolated snails that were submerged in water (and emerged from their shells) just prior to respiratory determinations showed a similar level of metabolic depression to ‘dry’ snails. This incidentally confirms that the determinations reflect cellular level metabolic processes rather than respiratory stress.
Evolutionary theory around the relationship between energy balance and fitness focuses on the maximization of energy gain when organisms are active, with fitness accumulating from one period of activity to the next [13]. This accords with the MTE's proposal of a uniform, resting metabolism by all organisms after scaling for mass and temperature [2,3]. In other words, because selective processes affect only active states, resting state metabolism of all organisms is assumed to be similar. In this context, hypometabolism of ectotherms during aestivation can be viewed as a means of extending the timeframe over which unfavourable conditions can be withstood. An additional approach to achieving energy balance is not only to maximize intake, but also to minimize loss and metabolic depression can be seen as a way of reducing the depletion of energy reserves. The evolutionary selection in Echinolittorina snails to maintain a positive energy status during inactivity through metabolic depression and thermal independence is strongly suggested by their constrained energy gain (feeding) in the face of extreme energy costs linked to the combination of high-temperature exposure, gastropod locomotion and shell production (see [15,33,34]). While energy is conserved through metabolic depression in aestivating snails during prolonged aerial exposure, it will also be conserved in recently rested non-aestivating snails when lower on the shore by rapid switching between thermal dependence and independence. By depressing metabolism when resting between tidal cycles, Echinolittorina snails enter subsequent feeding cycles with a greater energy surplus (accumulated over successive rest periods) than if metabolism was not depressed. These metabolic responses are especially relevant to the spatial and temporal complexity of the thermal environment of rocky shores (figure 1, [35,36]).
To summarize, this study highlights the complexity of metabolism in relation to temperature variation in resting Echinolittorina snails. The way in which metabolic rate varies with temperature depends on the temperature range experienced, the degree of metabolic depression, and whether snails are isolated within their shells. We suggest that this complexity is linked to the evolutionary selection of metabolic processes that conserve energy to overcome a lifetime constraint on energy gain (owing to prolonged inactivity) in a high cost environment characterized by solar-driven, fluctuating high temperatures [12,17]. These metabolic processes sometimes completely override Boltzmann kinetics and thus have critical implications for the mechanistic basis suggested by the UTD model. Furthermore, interpretations based on the model should be made with reservation in cases where the thermal niche of a species extends beyond the model's thermal frame and where organisms experience rapidly fluctuating high temperatures. As these circumstances are common and not confined to extremophiles living in restricted and unusual environments, the generality of the theory as a whole is in question.
Acknowledgements
This research was supported by the Ministry of Development and Universiti Brunei Darussalam, Brunei Darussalam (D.J.M.), and the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation (C.D.M.). We thank Innes Cuthill and three anonymous referees for suggestions that led to an improved manuscript.
References
- 1.Gillooly J. F., Brown J. H., West G. B., Savage V. M., Charnov E. L. 2001. Effects of size and temperature on metabolic rate. Science 293, 2248–2251 10.1126/science.1061967 (doi:10.1126/science.1061967) [DOI] [PubMed] [Google Scholar]
- 2.Brown J. H., Gillooly J. F., Allen A. P., Savage V. M., West G. B. 2004. Toward a metabolic theory of ecology. Ecology 85, 1771–1789 10.1890/03-9000 (doi:10.1890/03-9000) [DOI] [Google Scholar]
- 3.Gillooly J. F., Allen A. P., Savage V. M., Charnov E. L., West G. B., Brown J. H. 2006. Response to Clarke and Fraser: effects of temperature on metabolic rate. Funct. Ecol. 20, 400–404 10.1111/j.1365-2435.2006.01110.x (doi:10.1111/j.1365-2435.2006.01110.x) [DOI] [Google Scholar]
- 4.Allen A. P., Gillooly J. F. 2007. The mechanistic basis of the metabolic theory of ecology. Oikos 116, 1073–1077 10.1111/j.0030-1299.2007.16079.x (doi:10.1111/j.0030-1299.2007.16079.x) [DOI] [Google Scholar]
- 5.Clarke A. 2004. Is there a universal temperature dependence of metabolism? Funct. Ecol. 8, 252–256 [Google Scholar]
- 6.Clarke A. 2006. Temperature and the metabolic theory of ecology. Funct. Ecol. 20, 405–412 10.1111/j.1365-2435.2006.01109.x (doi:10.1111/j.1365-2435.2006.01109.x) [DOI] [Google Scholar]
- 7.O'Connor M. P., Kemp S. J., Agosta S. J., Hansen F., Sieg A. E., Wallace B. P., McNair J. N., Dunham A. E. 2007. Reconsidering the mechanistic basis of the metabolic theory of ecology. Oikos 116, 1058–1072 10.1111/j.0030-1299.2007.15534.x (doi:10.1111/j.0030-1299.2007.15534.x) [DOI] [Google Scholar]
- 8.Hochachka P. W., Somero G. N. 2002. Biochemical adaptation: mechanism and process in physiological evolution. Oxford, UK: Oxford University Press [Google Scholar]
- 9.Clarke A., Fraser K. P. P. 2004. Why does metabolism scale with temperature? Funct. Ecol. 18, 243–251 10.1111/j.0269-8463.2004.00841.x (doi:10.1111/j.0269-8463.2004.00841.x) [DOI] [Google Scholar]
- 10.Irlich U. M., Terblanche J. S., Blackburn T. M., Chown S. L. 2009. Insect rate-temperature relationships: environmental variation and the metabolic theory of ecology. Am. Nat. 174, 819–835 10.1086/647904 (doi:10.1086/647904) [DOI] [PubMed] [Google Scholar]
- 11.Terblanche J. S., Janion C., Chown S. L. 2007. Variation in scorpion metabolic rate and rate-temperature relationships: implications for the fundamental equation of the metabolic theory of ecology. J. Evol. Biol. 20, 1602–1612 10.1111/j.1420-9101.2007.01322.x (doi:10.1111/j.1420-9101.2007.01322.x) [DOI] [PubMed] [Google Scholar]
- 12.Marshall D. J., McQuaid C. D., Williams G. A. 2010. Non-climatic thermal adaptation: implications for species' responses to climate warming. Biol. Lett. (doi:10.1098/rsbl.2010.0233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilchrist G. W. 2000. The evolution of thermal sensitivity in changing environments. In Environmental stressors and gene response (eds Storey K. B., Storey J.), pp. 55–69 Amsterdam, The Netherlands: Elsevier Science BV [Google Scholar]
- 14.Angilletta M. J., Wilson R. S., Navas C. A., James R. S. 2003. Tradeoffs and the evolution of thermal reaction norms. Trends Ecol. Evol. 18, 234–240 10.1016/S0169-5347(03)00087-9 (doi:10.1016/S0169-5347(03)00087-9) [DOI] [Google Scholar]
- 15.Guppy M., Withers P. 1999. Metabolic depression in animals: physiological perspectives and biochemical generalizations. Biol. Rev. 74, 1–40 10.1017/S0006323198005258 (doi:10.1017/S0006323198005258) [DOI] [PubMed] [Google Scholar]
- 16.Artacho P., Nespolo R. F. 2009. Natural selection reduces energy metabolism in the garden snail, Helix aspera (Cornu aspersum). Evolution 63, 1044–1050 10.1111/j.1558-5646.2008.00603.x (doi:10.1111/j.1558-5646.2008.00603.x) [DOI] [PubMed] [Google Scholar]
- 17.Newell R. C. 1969. Effect of fluctuations in temperature on the metabolism of intertidal invertebrates. Am. Zool. 9, 293–307 [Google Scholar]
- 18.Percy J. A., Aldrich F. A. 1971. Metabolic rate independent of temperature in mollusc tissue. Nature 231, 393–394 10.1038/231393a0 (doi:10.1038/231393a0) [DOI] [PubMed] [Google Scholar]
- 19.Brown A. C., da Silva F. M. 1984. Effects of temperature on oxygen consumption in two closely-related whelks from different temperature regimes. J. Exp. Mar. Biol. Ecol. 84, 145–153 10.1016/0022-0981(84)90207-7 (doi:10.1016/0022-0981(84)90207-7) [DOI] [Google Scholar]
- 20.Marshall D. J., McQuaid C. D. 1991. Metabolic rate depression in a marine pulmonate snail: pre-adaptation for a terrestrial existence? Oecologia 88, 274–276 10.1007/BF00320822 (doi:10.1007/BF00320822) [DOI] [PubMed] [Google Scholar]
- 21.Sokolova I. M., Pörtner H. O. 2001. Physiological adaptations to high intertidal life involve improved water conservation abilities and metabolic rate depression in Littorina saxatilis. Mar. Ecol. Prog. Ser. 224, 71–86 [Google Scholar]
- 22.Hand S. C., Hardewig I. 1996. Downregulation of cellular metabolism during environmental stress: mechanisms and implications. Annu. Rev. Physiol. 58, 539–563 10.1146/annurev.ph.58.030196.002543 (doi:10.1146/annurev.ph.58.030196.002543) [DOI] [PubMed] [Google Scholar]
- 23.Storey K. B., Storey J. M. 2004. Metabolic rate depression in animals: transcriptional and translational controls. Biol. Rev. 79, 207–233 10.1017/S1464793103006195 (doi:10.1017/S1464793103006195) [DOI] [PubMed] [Google Scholar]
- 24.Marshall D. J., McQuaid C. D. 1992. Comparative aerial metabolism of the intertidal limpets Patella granularis L. (Mollusca: Prosobranchia) and Siphonaria oculus (Mollusca: Pulmonata). Physiol. Zool. 65, 1040–1056 [Google Scholar]
- 25.Houlihan D., Innes A. J. 1982. Respiration in air and water of four Mediterranean trochids. J. Exp. Mar. Biol. Ecol. 57, 35–54 10.1016/0022-0981(82)90143-5 (doi:10.1016/0022-0981(82)90143-5) [DOI] [Google Scholar]
- 26.Fretter V., Graham A. 1976. A functional anatomy of invertebrates. New York, NY: Academic Press [Google Scholar]
- 27.McMahon R. F. 1990. Thermal tolerance, evaporative water loss, air–oxygen consumption and zonation of intertidal prosobranchs: a new synthesis. Hydrobiologia 193, 241–260 10.1007/BF00028081 (doi:10.1007/BF00028081) [DOI] [Google Scholar]
- 28.Reid D. G. 2007. The genus Echinolittorina (Habe, 1956) (Gastropoda: Littorinidae) in the Indo-West Pacific Ocean. Zootaxa 1420, 1–161 [Google Scholar]
- 29.Sokolova I. M., Pörtner H. O. 2003. Metabolic plasticity and critical temperatures for aerobic scope in a eurythermal marine invertebrate (Littorina saxatilis, Gastropoda: Littorinidae) from different latitudes. J. Exp. Biol. 206, 195–207 10.1242/jeb.00054 (doi:10.1242/jeb.00054) [DOI] [PubMed] [Google Scholar]
- 30.McMahon R. F., Russell-Hunter W. D., Aldridge D. W. 1995. Lack of metabolic temperature compensation in the intertidal gastropods, Littorina saxatilis (Olivi) and L. obtusata (L.). Hydrobiologia 309, 89–100 10.1007/BF00014475 (doi:10.1007/BF00014475) [DOI] [Google Scholar]
- 31.Quinn G. P., Keough M. J. 2002. Experimental design and data analysis for biologists. Cambridge, UK: Cambridge University Press [Google Scholar]
- 32.Denny M. W., Harley C. D. G. 2006. Hot limpets: predicting body temperatures in a conductance-mediated thermal system. J. Exp. Biol. 209, 2409–2419 10.1242/jeb.02257 (doi:10.1242/jeb.02257) [DOI] [PubMed] [Google Scholar]
- 33.Bochdansky A., Gronkjaer P., Herra T., Leggett W. 2005. Experimental evidence for selection against fish larva with high metabolic rate in a food limited environment. Mar. Biol. 47, 1413–1417 [Google Scholar]
- 34.Parsons P. A. 2005. Environments and evolution: interactions between stress, resource inadequacy and energetic efficiency. Biol. Rev. 80, 589–610 10.1017/S1464793105006822 (doi:10.1017/S1464793105006822) [DOI] [PubMed] [Google Scholar]
- 35.Helmuth B., Kingsolver J. G., Carrington E. 2005. Biophysics, physiological ecology, and climate change: does mechanism matter? Annu. Rev. Physiol. 67, 177–201 10.1146/annurev.physiol.67.040403.105027 (doi:10.1146/annurev.physiol.67.040403.105027) [DOI] [PubMed] [Google Scholar]
- 36.Gilman S. E., Wethey D. S., Helmuth B. 2006. Variation in the sensitivity of organismal body temperature to climate change over local and geographic scales. Proc. Natl Acad. Sci. USA 103, 9560–9565 10.1073/pnas.0510992103 (doi:10.1073/pnas.0510992103) [DOI] [PMC free article] [PubMed] [Google Scholar]